The `mosdef` User's Guide
Leon Dammer
Institute of Medical Biostatistics, Epidemiology and Informatics (IMBEI), Mainzdammerle@uni-mainz.de
Federico Marini
Institute of Medical Biostatistics, Epidemiology and Informatics (IMBEI), MainzResearch Center for Immunotherapy (FZI), Mainzmarinif@uni-mainz.de
15 May 2025
Source:vignettes/mosdef_userguide.Rmd
mosdef_userguide.RmdIntroduction
This vignette describes how to use the mosdef package for performing tasks commonly associated to your Differential Expression workflows.
This includes functionality for
plotting your expression values and differential expression results, both individually and as summary overviews (
gene_plot,de_volcano,go_volcano,plot_ma,get_expr_values)running different methods for functional enrichment analysis, providing a unified API that simplifies the calls to the individual methods, ensuring the results are also provided in a standardized format (
run_cluPro,run_topGO,run_goseq)decorating and improving your analysis reports (assuming these are generated as Rmarkdown documents). This can enhance the experience of browsing the results by automatical linking to external databases (ENSEMBL, GTEX, HPA, NCBI, … via
create_link_functions, wrapped into abuttonifierto seamlessly multiply the information in a simple tabular representation). Additional info on frequently used features such as genes and Gene Ontology terms can also be embedded withgeneinfo_to_htmlandgo_to_html
The mosdef package as a whole aims to collect the MOSt frequently used DE-related Functions, and is open to further contributions from the community.
All in all, the objective for mosdef is to streamline the generation of comprehensive, information-rich analysis reports.
Historically, many of these functions (at least conceptually) have been developed in some of our other Bioconductor packages, such as pcaExplorer, ideal and GeneTonic. mosdef is the attempt to achieve a better modularization for the most common tasks in the DE workflow.
Required input
In order to use mosdef in your workflow, two main inputs are required:
-
de_container, a dataset containing the expression matrix. For example, aDESeqDataSetobject in the DESeq2 workflow -
res_de, a dataset, such as aDESeqResultsstoring the results of the differential expression analysis
Additionally, the mapping parameters, shared by a number
of functions, refers to the annotation of your species provided by
AnnotationDbi-like
packages, which are commonplace in the Bioconductor environment. For
human, this would be org.Hs.eg.db,
and for mouse org.Mm.eg.db.
Currently, mosdef is able to feed on the classes used throughout the DESeq2 approach, but can easily be extended for the corresponding implementations in edgeR and limma.
Demonstrating mosdef on the
macrophage data
In the remainder of this vignette, we will illustrate the main features of mosdef on a publicly available dataset from Alasoo et al., “Shared genetic effects on chromatin and gene expression indicate a role for enhancer priming in immune response”, published in Nature Genetics, January 2018 (Alasoo et al. 2018) doi:10.1038/s41588-018-0046-7.
The data is made available via the macrophage Bioconductor package, which contains the files output from the Salmon quantification (version 0.12.0, with Gencode v29 reference), as well as the values summarized at the gene level, which we will use to exemplify the analysis steps.
In the macrophage experimental setting, the samples are
available from 6 different donors, in 4 different conditions (naive,
treated with Interferon gamma, with SL1344, or with a combination of
Interferon gamma and SL1344). For simplicity, we will restrict our
attention on the comparison between Interferon gamma treated samples vs
naive samples.
Installation
To install mosdef, you can run the following commands:
if (!require("BiocManager")) {
install.packages("BiocManager")
}
BiocManager::install("mosdef")If you want to install the development version from GitHub, you can alternatively run this command:
BiocManager::install("imbeimainz/mosdef")Getting started
Once installed, the mosdef package can be loaded and attached to your current workspace as follows:
Load the data
We will use the well known macrophage data as an
example.
Notably, we correctly specify the experimental design as
~ line + condition, to obtain the effect due to the
condition, while accounting for the cell
line.
If you are familiar with the DE workflow, you can skim over this section and read more on the functionality of mosdef in Section @ref(mosdefenrich).
suppressPackageStartupMessages({
library("macrophage")
library("DESeq2")
library("org.Hs.eg.db")
})
data(gse, package = "macrophage")
dds_macrophage <- DESeqDataSet(gse, design = ~ line + condition)
#> using counts and average transcript lengths from tximeta
rownames(dds_macrophage) <- substr(rownames(dds_macrophage), 1, 15)We perform some filtering on the features to be kept, and define the
set of differentially expressed genes contrasting the IFNg
and the naive samples.
Notably, we correctly specify the lfcThreshold parameter
instead of a post-hoc approach to filter the DE table based on the log2
fold change values - see https://support.bioconductor.org/p/101504/ for an
excellent explanation on why to prefer the more rigorous (yet, likely
conservative) method defined in the chunk below.
keep <- rowSums(counts(dds_macrophage) >= 10) >= 6
dds_macrophage <- dds_macrophage[keep, ]
dds_macrophage <- DESeq(dds_macrophage)
#> estimating size factors
#> using 'avgTxLength' from assays(dds), correcting for library size
#> estimating dispersions
#> gene-wise dispersion estimates
#> mean-dispersion relationship
#> final dispersion estimates
#> fitting model and testing
res_macrophage_IFNg_vs_naive <- results(dds_macrophage,
contrast = c("condition", "IFNg", "naive"),
lfcThreshold = 1,
alpha = 0.05)Please refer to the vignette of the DESeq2 or
edgeR
packages for more complex experimental designs and/or additional options
in each workflow.
The aim for this section was simply to generate exemplary objects to
work with and provide to the mosdef
functions.
Generating enrichment results with a unified API
mosdef
allows you to create your enrichment results right from your
DESeqDataset and DESeqResults objects using 3
possible algorithms, widely used:
For more information on the differences between these algorithms we refer to their individual vignettes and publications.
All of these algorithms require an annotation to function properly,
so make sure you have installed and use the correct one for your
experimental data. The default is org.Mm.eg.db (Mus
musculus). The macrophage data however stems from human, so
we need org.Hs.eg.db, and we load this package in the
following chunk:
library("AnnotationDbi")
library("org.Hs.eg.db")We also want to add a symbol column for later use - and, in order to add a human readable name for our features of interest:
res_macrophage_IFNg_vs_naive$symbol <-
AnnotationDbi::mapIds(org.Hs.eg.db,
keys = row.names(res_macrophage_IFNg_vs_naive),
column = "SYMBOL",
keytype = "ENSEMBL",
multiVals = "first"
)
#> 'select()' returned 1:many mapping between keys and columnsWe indeed recommend to use identifiers as row names that are
machine-readable and stable over time, such as ENSEMBL or GENCODE.
To ensure that we are using objects that would work out-of-the-box into
mosdef, we
provide some utilities to check that in advance - this can relax the
need of specifying a number of parameters in the other functions.
mosdef_de_container_check(dds_macrophage)
mosdef_res_check(res_macrophage_IFNg_vs_naive)
mosdef and topGO
topGO is a widely used option to obtain a set of spot-on Gene Ontology terms, removing some of the more generic ones and therefore also reducing the redundancy which is inherent in the GO database (Ashburner et al. 2000).
library("topGO")
#> Loading required package: graph
#> Loading required package: GO.db
#> Loading required package: SparseM
#>
#> groupGOTerms: GOBPTerm, GOMFTerm, GOCCTerm environments built.
#>
#> Attaching package: 'topGO'
#> The following object is masked from 'package:IRanges':
#>
#> members
res_enrich_macrophage_topGO <- run_topGO(
de_container = dds_macrophage,
res_de = res_macrophage_IFNg_vs_naive,
ontology = "BP",
mapping = "org.Hs.eg.db",
FDR_threshold = 0.05,
gene_id = "symbol",
de_type = "up_and_down",
add_gene_to_terms = TRUE,
topGO_method2 = "elim",
min_counts = 20,
top_de = 700,
verbose = TRUE
)
#> 'select()' returned 1:many mapping between keys and columns
#> 'select()' returned 1:many mapping between keys and columns
#> Your dataset has 1024 DE genes.
#> You selected 700 (68.36%) genes for the enrichment analysis.
#> You are analyzing up_and_down-regulated genes in the `res_de` container
#> Warning in run_topGO(de_container = dds_macrophage, res_de =
#> res_macrophage_IFNg_vs_naive, : NAs introduced by coercion
#> 6071 GO terms were analyzed. Not all of them are significantly enriched.
#> We suggest further subsetting the output list by for example:
#> using a pvalue cutoff in the column:
#> 'p.value_elim'.The run_topGO function will return a table with the
analysis for all possible GO terms (currently when using the “BP”
ontology on the macrophage dataset that is 6071 terms). Not all of these
results are significant, and this list can (should) be further
subset/filtered. For example by using a p-value cutoff.
The key parameters for run_topGO() are defined as
follows:
-
de_container: YourDESeqDatasetobject -
res_de: YourDESeqResultsobject -
ontology: Which gene ontology to analyse, default is “BP” -
mapping: The annotation/species -
gene_id: Which format the genes are provided. If you provide aDESeqDatasetandDESeqResults, then mosdef does it for you and uses symbols. If you provide vectors please specify a value. -
FDR_threshold: The pvalue to use to count a gene as significant. The default is 0.05 but if you want a stricter analysis you could set this to 0.01 for example -
de_type: Which genes to analyse. The default is all (“up_and_down”) Other possibilities are only up-/down-regulated (“up”/“down”) genes. -
add_gene_to_terms: Logical, whether to add a column with all genes annotated to each GO term. -
topGO_method2: Character, specifying which of the methods implemented bytopGOis to be used. The default is elim. For more info look at the documentation of topGO. -
min_counts: Minimum number of counts a gene has to have to be considered for the background. The default is 0 and we advise this parameter is only used by expert users that understand the impact of selecting “non-standard” backgrounds. -
top_de: The number of genes to be considered in the enrich analysis. The default is all genes, this can be reduced to reduce redundancy. In this case, we take the top 700 highest DE genes based of padj score. If this number is bigger than the total amount of de genes the parameter defaults back to all genes. -
verbose: Whether or not to summarise the analysis in a message.
head(res_enrich_macrophage_topGO)
#> GO.ID
#> 1 GO:0002250
#> 2 GO:0002503
#> 3 GO:0019886
#> 4 GO:0045087
#> 5 GO:0034341
#> 6 GO:0001916
#> Term
#> 1 adaptive immune response
#> 2 peptide antigen assembly with MHC class II protein complex
#> 3 antigen processing and presentation of exogenous peptide antigen via MHC class II
#> 4 innate immune response
#> 5 response to type II interferon
#> 6 positive regulation of T cell mediated cytotoxicity
#> Annotated Significant Expected Rank in p.value_classic p.value_elim
#> 1 384 89 15.97 3 4.0e-21
#> 2 13 13 0.54 26 9.5e-19
#> 3 27 15 1.12 53 1.7e-14
#> 4 784 104 32.61 16 1.6e-11
#> 5 110 28 4.58 49 5.2e-11
#> 6 31 13 1.29 102 9.8e-11
#> p.value_classic
#> 1 NA
#> 2 9.7e-19
#> 3 1.7e-14
#> 4 3.2e-27
#> 5 4.9e-15
#> 6 1.0e-10
#> genes
#> 1 ASCL2,B2M,BTN3A1,BTN3A2,BTN3A3,C1QB,C1RL,C1S,C2,C3,C4A,C4B,CD1A,CD274,CD28,CD40,CD7,CD74,CD80,CEACAM1,CLEC10A,CLEC6A,CR1L,CSF2RB,CTLA4,CTSS,CX3CR1,ERAP2,EXO1,FGL1,GPR183,HLA-A,HLA-B,HLA-C,HLA-DMA,HLA-DMB,HLA-DOA,HLA-DOB,HLA-DPA1,HLA-DPB1,HLA-DQA1,HLA-DQB1,HLA-DQB2,HLA-DRA,HLA-DRB1,HLA-DRB5,HLA-E,HLA-F,HLA-H,ICAM1,IL12RB1,IL18BP,IL27,IL4I1,IRF1,IRF7,ITK,JAK2,JAK3,KLRK1,LAG3,LAMP3,LILRA1,LILRB3,MCOLN2,NOD2,P2RX7,PCYT1A,PDCD1,PDCD1LG2,RIPK2,RNF19B,RSAD2,SECTM1,SERPING1,SIT1,SLAMF1,SLAMF6,SLAMF7,SLC11A1,TAP1,TAP2,TBX21,TLR8,TNFRSF11A,TNFRSF21,TNFSF13B,TNFSF18,ZC3H12A
#> 2 B2M,HLA-DMA,HLA-DMB,HLA-DOA,HLA-DOB,HLA-DPA1,HLA-DPB1,HLA-DQA1,HLA-DQB1,HLA-DQB2,HLA-DRA,HLA-DRB1,HLA-DRB5
#> 3 B2M,CD74,CTSS,HLA-DMA,HLA-DMB,HLA-DOA,HLA-DOB,HLA-DPA1,HLA-DPB1,HLA-DQA1,HLA-DQB1,HLA-DQB2,HLA-DRA,HLA-DRB1,HLA-DRB5
#> 4 ACOD1,ADAM8,AIM2,APOBEC3A,APOBEC3D,APOBEC3G,APOL1,B2M,C1QB,C1RL,C1S,C2,C3,C4A,C4B,CALCOCO2,CALHM6,CASP4,CD274,CD300H,CD300LF,CD40,CD74,CEACAM1,CFB,CFH,CIITA,CLEC10A,CLEC6A,COLEC12,CTSS,CX3CR1,CXCL10,CYLD,DTX3L,EDN1,GBP1,GBP2,GBP3,GBP4,GBP5,GBP6,GBP7,GCH1,GSDMD,H2BC21,HLA-A,HLA-B,HLA-C,HLA-DPA1,HLA-E,HLA-F,IFI27,IFI35,IFIH1,IFIT2,IFIT3,IFITM1,IFITM2,IL12RB1,IL27,IRF1,IRF7,ISG20,JAK2,JAK3,KLRK1,LAG3,LILRA2,MCOLN2,MEFV,MSRB1,NCF1,NLRC5,NMI,NOD2,NUB1,OPTN,P2RX7,PIM1,PML,RAB20,RIPK2,RNF19B,RSAD2,SCIMP,SERPING1,SLAMF1,SLAMF6,SLAMF7,SLC11A1,STAT1,STAT2,TIFA,TLR10,TLR8,TRAFD1,TRIM17,TRIM22,TRIM69,UBD,UBE2L6,ZBP1,ZNFX1
#> 5 ACOD1,CALCOCO2,CD40,CD74,CIITA,EDN1,GBP1,GBP2,GBP3,GBP4,GBP5,GBP6,GBP7,GCH1,HLA-DPA1,IFITM1,IFITM2,IL12RB1,IRF1,JAK2,MEFV,NLRC5,NUB1,PIM1,RAB20,SLC11A1,STAT1,UBD
#> 6 B2M,CD1A,HLA-A,HLA-B,HLA-C,HLA-DRA,HLA-DRB1,HLA-E,HLA-F,HLA-H,IL12RB1,P2RX7,TAP2This will return a table with 9 columns. They contain:
-
GO.ID: The computer readable GO term ID. -
Term: The human readable GO term name. -
Annotated: The overall number of detected genes associated with this term (as defined in thede_containeror in thebg_genesparameters). -
Significant: The number of DE genes associated with this term (as defined in theres_deor in thede_genesparameters) -
Expected: The expected number of genes associated with this term. -
Rank in p.value_classic: specifies the rank of each term, if using the ascending order ofp.value_classicto sort the entries. -
p.value_elim: p-value for the enrichment test of each term (recommended for sorting the table), based on theelimalgorithm intopGO. -
p.value_classic: p-value for the enrichment, using the “classical” Fisher test. -
genes: Specifies which DE genes are associated with that term (separated by a comma). This column will later on be used to label the genes in thego_volcano()function (see below in Section @ref(volcanoplots))
mosdef and goseq
The method implemented in the goseq package is able to handle the selection bias inherent in assays such as RNA-seq, whereas highly expressed genes have a higher probability of being detected as differentially expressed.
Parameters like top_de, min_counts,
verbose, FDR_threshold and
de_type can also be used here (for more detail on these
parameters see above), as they are a part of the shared API with
run_topGO and run_cluPro.
Importantly, the feature length retrieval is based on the
goseq() function, and this requires that the corresponding
TxDb packages are installed and available. So make sure one is installed
on your machine. For human samples, the recommended one is TxDb.Hsapiens.UCSC.hg38.knownGene.
goseq_macrophage <- run_goseq(
de_container = dds_macrophage,
res_de = res_macrophage_IFNg_vs_naive,
mapping = "org.Hs.eg.db",
testCats = "GO:BP" # which categories to test of ("GO:BP, "GO:MF", "GO:CC")
)
#> Your dataset has 1024 DE genes.
#> You selected 1024 (100.00%) genes for the enrichment analysis.
#> You are analyzing up_and_down-regulated genes in the `res_de` container
#> Can't find hg38/ensGene length data in genLenDataBase...
#> Warning in grep(txdbPattern, installedPackages): argument 'pattern' has length
#> > 1 and only the first element will be used
#> Found the annotation package, TxDb.Hsapiens.UCSC.hg38.knownGene
#> Trying to get the gene lengths from it.
#> Loading required package: GenomicFeatures
#>
#> Attaching package: 'GenomicFeatures'
#> The following object is masked from 'package:topGO':
#>
#> genes
#> Warning in pcls(G): initial point very close to some inequality constraints
#> Fetching GO annotations...
#> For 3710 genes, we could not find any categories. These genes will be excluded.
#> To force their use, please run with use_genes_without_cat=TRUE (see documentation).
#> This was the default behavior for version 1.15.1 and earlier.
#> Calculating the p-values...
#> 'select()' returned 1:1 mapping between keys and columns
#> 'select()' returned 1:many mapping between keys and columns
head(goseq_macrophage)
#> category over_represented_pvalue under_represented_pvalue numDEInCat
#> 1766 GO:0006955 4.037493e-55 1 240
#> 610 GO:0002376 1.618960e-49 1 291
#> 523 GO:0002250 4.897194e-41 1 104
#> 2392 GO:0009607 1.552951e-39 1 197
#> 8181 GO:0051707 2.869334e-39 1 193
#> 6262 GO:0043207 3.258071e-39 1 193
#> numInCat term ontology p.adj
#> 1766 1427 immune response BP 5.854365e-51
#> 610 2102 immune system process BP 1.173746e-45
#> 523 385 adaptive immune response BP 2.366977e-37
#> 2392 1257 response to biotic stimulus BP 5.629447e-36
#> 8181 1220 response to other organism BP 7.873671e-36
#> 6262 1221 response to external biotic stimulus BP 7.873671e-36
#> genes
#> 1766 ENSG00000125347,ENSG00000162645,ENSG00000145365,ENSG00000137496,ENSG00000154451,ENSG00000231389,ENSG00000204257,ENSG00000100342,ENSG00000162654,ENSG00000164509,ENSG00000204267,ENSG00000131203,ENSG00000213886,ENSG00000168394,ENSG00000242574,ENSG00000163568,ENSG00000183734,ENSG00000101017,ENSG00000117228,ENSG00000244731,ENSG00000019582,ENSG00000204252,ENSG00000089041,ENSG00000179583,ENSG00000136436,ENSG00000188820,ENSG00000120217,ENSG00000204287,ENSG00000131979,ENSG00000096996,ENSG00000113263,ENSG00000169248,ENSG00000117226,ENSG00000026751,ENSG00000224389,ENSG00000004468,ENSG00000197646,ENSG00000164136,ENSG00000149131,ENSG00000008517,ENSG00000089692,ENSG00000184588,ENSG00000078081,ENSG00000223865,ENSG00000073861,ENSG00000164308,ENSG00000173369,ENSG00000169245,ENSG00000196126,ENSG00000167207,ENSG00000204642,ENSG00000079385,ENSG00000123146,ENSG00000179344,ENSG00000181374,ENSG00000186470,ENSG00000198502,ENSG00000234745,ENSG00000000971,ENSG00000152207,ENSG00000213809,ENSG00000140853,ENSG00000121858,ENSG00000243649,ENSG00000026950,ENSG00000206341,ENSG00000204592,ENSG00000102794,ENSG00000141574,ENSG00000153064,ENSG00000138755,ENSG00000172183,ENSG00000141655,ENSG00000206503,ENSG00000123609,ENSG00000123240,ENSG00000115415,ENSG00000213512,ENSG00000163599,ENSG00000162931,ENSG00000204525,ENSG00000198736,ENSG00000096968,ENSG00000198829,ENSG00000168329,ENSG00000205220,ENSG00000127951,ENSG00000013374,ENSG00000151651,ENSG00000137193,ENSG00000135148,ENSG00000232629,ENSG00000158270,ENSG00000197721,ENSG00000165949,ENSG00000173762,ENSG00000104312,ENSG00000101916,ENSG00000156587,ENSG00000111801,ENSG00000104951,ENSG00000166278,ENSG00000102524,ENSG00000018280,ENSG00000170581,ENSG00000108688,ENSG00000169508,ENSG00000197272,ENSG00000026103,ENSG00000068079,ENSG00000140749,ENSG00000178562,ENSG00000153898,ENSG00000241106,ENSG00000196735,ENSG00000185201,ENSG00000090339,ENSG00000284690,ENSG00000205846,ENSG00000275718,ENSG00000243811,ENSG00000124256,ENSG00000204577,ENSG00000184678,ENSG00000185885,ENSG00000120337,ENSG00000121594,ENSG00000104518,ENSG00000188389,ENSG00000132274,ENSG00000140464,ENSG00000100368,ENSG00000146072,ENSG00000116514,ENSG00000166710,ENSG00000108700,ENSG00000128383,ENSG00000125730,ENSG00000162739,ENSG00000239713,ENSG00000132514,ENSG00000186074,ENSG00000103313,ENSG00000161929,ENSG00000189171,ENSG00000119917,ENSG00000174123,ENSG00000163874,ENSG00000121797,ENSG00000163131,ENSG00000105639,ENSG00000083799,ENSG00000104760,ENSG00000203805,ENSG00000119922,ENSG00000196954,ENSG00000035720,ENSG00000158477,ENSG00000124201,ENSG00000139832,ENSG00000174371,ENSG00000185507,ENSG00000185880,ENSG00000134321,ENSG00000104974,ENSG00000120436,ENSG00000137959,ENSG00000136960,ENSG00000173198,ENSG00000124508,ENSG00000182326,ENSG00000078401,ENSG00000139178,ENSG00000115267,ENSG00000100226,ENSG00000137078,ENSG00000117090,ENSG00000158517,ENSG00000163840,ENSG00000183347,ENSG00000113749,ENSG00000239998,ENSG00000186810,ENSG00000136689,ENSG00000161217,ENSG00000064932,ENSG00000054219,ENSG00000204616,ENSG00000137628,ENSG00000135636,ENSG00000159403,ENSG00000142089,ENSG00000110848,ENSG00000065427,ENSG00000075240,ENSG00000204632,ENSG00000082074,ENSG00000158714,ENSG00000158481,ENSG00000158813,ENSG00000187554,ENSG00000171522,ENSG00000237541,ENSG00000099985,ENSG00000104856,ENSG00000113916,ENSG00000275385,ENSG00000189067,ENSG00000178999,ENSG00000271503,ENSG00000254087,ENSG00000106952,ENSG00000168811,ENSG00000111335,ENSG00000163735,ENSG00000255833,ENSG00000163564,ENSG00000152778,ENSG00000164300,ENSG00000136044,ENSG00000173193,ENSG00000198719,ENSG00000196664,ENSG00000085265,ENSG00000243414,ENSG00000076248,ENSG00000184371,ENSG00000226979,ENSG00000119508,ENSG00000157873,ENSG00000136560,ENSG00000041880,ENSG00000111331,ENSG00000138496,ENSG00000104432,ENSG00000135047,ENSG00000092445,ENSG00000188313,ENSG00000112149,ENSG00000137265
#> 610 ENSG00000125347,ENSG00000162645,ENSG00000145365,ENSG00000137496,ENSG00000154451,ENSG00000231389,ENSG00000204257,ENSG00000100342,ENSG00000162654,ENSG00000134470,ENSG00000164509,ENSG00000204267,ENSG00000131203,ENSG00000213886,ENSG00000168394,ENSG00000242574,ENSG00000163568,ENSG00000183734,ENSG00000101017,ENSG00000117228,ENSG00000244731,ENSG00000019582,ENSG00000204252,ENSG00000089041,ENSG00000179583,ENSG00000136436,ENSG00000170989,ENSG00000185338,ENSG00000188820,ENSG00000120217,ENSG00000188906,ENSG00000204287,ENSG00000131979,ENSG00000096996,ENSG00000113263,ENSG00000169248,ENSG00000117226,ENSG00000026751,ENSG00000240065,ENSG00000224389,ENSG00000004468,ENSG00000197646,ENSG00000164136,ENSG00000149131,ENSG00000008517,ENSG00000089692,ENSG00000184588,ENSG00000078081,ENSG00000223865,ENSG00000073861,ENSG00000164308,ENSG00000173369,ENSG00000169245,ENSG00000196126,ENSG00000167207,ENSG00000110852,ENSG00000204642,ENSG00000079385,ENSG00000123146,ENSG00000179344,ENSG00000181374,ENSG00000186470,ENSG00000198502,ENSG00000234745,ENSG00000000971,ENSG00000152207,ENSG00000188404,ENSG00000213809,ENSG00000140853,ENSG00000121858,ENSG00000092010,ENSG00000243649,ENSG00000026950,ENSG00000206341,ENSG00000204592,ENSG00000102794,ENSG00000141574,ENSG00000168062,ENSG00000153064,ENSG00000138755,ENSG00000172183,ENSG00000141655,ENSG00000206503,ENSG00000123609,ENSG00000188676,ENSG00000117115,ENSG00000123240,ENSG00000115415,ENSG00000213512,ENSG00000163599,ENSG00000162931,ENSG00000204525,ENSG00000182782,ENSG00000198736,ENSG00000096968,ENSG00000198829,ENSG00000168329,ENSG00000205220,ENSG00000127951,ENSG00000013374,ENSG00000002933,ENSG00000151651,ENSG00000137193,ENSG00000135148,ENSG00000135424,ENSG00000232629,ENSG00000158270,ENSG00000197721,ENSG00000106565,ENSG00000165949,ENSG00000173762,ENSG00000088992,ENSG00000104312,ENSG00000101916,ENSG00000156587,ENSG00000111801,ENSG00000104951,ENSG00000123685,ENSG00000166278,ENSG00000102524,ENSG00000018280,ENSG00000170581,ENSG00000182580,ENSG00000108688,ENSG00000169508,ENSG00000231925,ENSG00000197272,ENSG00000026103,ENSG00000204264,ENSG00000068079,ENSG00000140749,ENSG00000178562,ENSG00000153898,ENSG00000241106,ENSG00000196735,ENSG00000162692,ENSG00000185201,ENSG00000090339,ENSG00000049130,ENSG00000099377,ENSG00000284690,ENSG00000153094,ENSG00000205846,ENSG00000275718,ENSG00000243811,ENSG00000124256,ENSG00000204577,ENSG00000184678,ENSG00000185885,ENSG00000090554,ENSG00000120337,ENSG00000121594,ENSG00000104518,ENSG00000188389,ENSG00000162367,ENSG00000116016,ENSG00000064201,ENSG00000132274,ENSG00000140464,ENSG00000100368,ENSG00000146072,ENSG00000116514,ENSG00000173821,ENSG00000166710,ENSG00000108700,ENSG00000128383,ENSG00000125730,ENSG00000162739,ENSG00000239713,ENSG00000132514,ENSG00000186074,ENSG00000103313,ENSG00000161929,ENSG00000189171,ENSG00000119917,ENSG00000174123,ENSG00000163874,ENSG00000121797,ENSG00000163131,ENSG00000105639,ENSG00000083799,ENSG00000104760,ENSG00000135124,ENSG00000133800,ENSG00000203805,ENSG00000119922,ENSG00000196954,ENSG00000023330,ENSG00000035720,ENSG00000158477,ENSG00000124201,ENSG00000139832,ENSG00000174371,ENSG00000006747,ENSG00000185507,ENSG00000119969,ENSG00000185880,ENSG00000134321,ENSG00000125810,ENSG00000162337,ENSG00000104974,ENSG00000120436,ENSG00000137959,ENSG00000136960,ENSG00000173198,ENSG00000170271,ENSG00000124508,ENSG00000182326,ENSG00000078401,ENSG00000139178,ENSG00000150630,ENSG00000115267,ENSG00000100226,ENSG00000137078,ENSG00000117090,ENSG00000158517,ENSG00000163840,ENSG00000183347,ENSG00000141682,ENSG00000113749,ENSG00000239998,ENSG00000186810,ENSG00000134256,ENSG00000136689,ENSG00000161217,ENSG00000139192,ENSG00000064932,ENSG00000054219,ENSG00000204616,ENSG00000137628,ENSG00000135636,ENSG00000159403,ENSG00000154639,ENSG00000142089,ENSG00000110848,ENSG00000065427,ENSG00000075240,ENSG00000204632,ENSG00000100628,ENSG00000082074,ENSG00000158714,ENSG00000070501,ENSG00000158481,ENSG00000158813,ENSG00000187554,ENSG00000146918,ENSG00000171522,ENSG00000237541,ENSG00000099985,ENSG00000104856,ENSG00000113916,ENSG00000275385,ENSG00000189067,ENSG00000178999,ENSG00000271503,ENSG00000254087,ENSG00000106952,ENSG00000168811,ENSG00000196684,ENSG00000111335,ENSG00000163735,ENSG00000255833,ENSG00000163564,ENSG00000152778,ENSG00000164300,ENSG00000123610,ENSG00000136044,ENSG00000173193,ENSG00000198719,ENSG00000196664,ENSG00000085265,ENSG00000142405,ENSG00000243414,ENSG00000076248,ENSG00000184371,ENSG00000226979,ENSG00000113249,ENSG00000119508,ENSG00000157873,ENSG00000136560,ENSG00000041880,ENSG00000111331,ENSG00000138496,ENSG00000153208,ENSG00000104432,ENSG00000135047,ENSG00000129422,ENSG00000092445,ENSG00000188313,ENSG00000112149,ENSG00000137265
#> 523 ENSG00000125347,ENSG00000137496,ENSG00000231389,ENSG00000204257,ENSG00000204267,ENSG00000168394,ENSG00000242574,ENSG00000183734,ENSG00000101017,ENSG00000244731,ENSG00000019582,ENSG00000204252,ENSG00000089041,ENSG00000120217,ENSG00000204287,ENSG00000096996,ENSG00000113263,ENSG00000026751,ENSG00000224389,ENSG00000197646,ENSG00000149131,ENSG00000089692,ENSG00000078081,ENSG00000223865,ENSG00000073861,ENSG00000164308,ENSG00000173369,ENSG00000196126,ENSG00000167207,ENSG00000204642,ENSG00000079385,ENSG00000179344,ENSG00000186470,ENSG00000198502,ENSG00000234745,ENSG00000213809,ENSG00000026950,ENSG00000206341,ENSG00000204592,ENSG00000141574,ENSG00000141655,ENSG00000206503,ENSG00000163599,ENSG00000204525,ENSG00000096968,ENSG00000168329,ENSG00000232629,ENSG00000197721,ENSG00000173762,ENSG00000104312,ENSG00000101916,ENSG00000111801,ENSG00000104951,ENSG00000166278,ENSG00000102524,ENSG00000018280,ENSG00000169508,ENSG00000197272,ENSG00000178562,ENSG00000153898,ENSG00000241106,ENSG00000196735,ENSG00000090339,ENSG00000205846,ENSG00000204577,ENSG00000120337,ENSG00000121594,ENSG00000188389,ENSG00000100368,ENSG00000146072,ENSG00000116514,ENSG00000166710,ENSG00000125730,ENSG00000162739,ENSG00000132514,ENSG00000163874,ENSG00000163131,ENSG00000105639,ENSG00000104760,ENSG00000158477,ENSG00000174371,ENSG00000185507,ENSG00000134321,ENSG00000104974,ENSG00000182326,ENSG00000139178,ENSG00000137078,ENSG00000117090,ENSG00000161217,ENSG00000159403,ENSG00000110848,ENSG00000204632,ENSG00000158481,ENSG00000237541,ENSG00000104856,ENSG00000113916,ENSG00000254087,ENSG00000168811,ENSG00000076248,ENSG00000226979,ENSG00000157873,ENSG00000041880,ENSG00000135047,ENSG00000137265
#> 2392 ENSG00000125347,ENSG00000162645,ENSG00000145365,ENSG00000137496,ENSG00000154451,ENSG00000231389,ENSG00000100342,ENSG00000162654,ENSG00000164509,ENSG00000204267,ENSG00000131203,ENSG00000213886,ENSG00000163568,ENSG00000101017,ENSG00000117228,ENSG00000244731,ENSG00000019582,ENSG00000089041,ENSG00000179583,ENSG00000136436,ENSG00000188820,ENSG00000120217,ENSG00000131979,ENSG00000096996,ENSG00000169248,ENSG00000117226,ENSG00000026751,ENSG00000224389,ENSG00000197646,ENSG00000164136,ENSG00000149131,ENSG00000186439,ENSG00000089692,ENSG00000184588,ENSG00000073861,ENSG00000173369,ENSG00000169245,ENSG00000196126,ENSG00000167207,ENSG00000204642,ENSG00000079385,ENSG00000181374,ENSG00000234745,ENSG00000000971,ENSG00000213809,ENSG00000140853,ENSG00000243649,ENSG00000204592,ENSG00000102794,ENSG00000168062,ENSG00000153064,ENSG00000138755,ENSG00000172183,ENSG00000141655,ENSG00000206503,ENSG00000123609,ENSG00000123240,ENSG00000115415,ENSG00000213512,ENSG00000162931,ENSG00000204525,ENSG00000198736,ENSG00000096968,ENSG00000168329,ENSG00000127951,ENSG00000013374,ENSG00000151651,ENSG00000137193,ENSG00000135148,ENSG00000178726,ENSG00000158270,ENSG00000165949,ENSG00000104312,ENSG00000176485,ENSG00000101916,ENSG00000156587,ENSG00000104951,ENSG00000123685,ENSG00000166278,ENSG00000018280,ENSG00000170581,ENSG00000108688,ENSG00000197272,ENSG00000101412,ENSG00000068079,ENSG00000153898,ENSG00000162692,ENSG00000140465,ENSG00000185201,ENSG00000284690,ENSG00000136514,ENSG00000136826,ENSG00000205846,ENSG00000275718,ENSG00000243811,ENSG00000124256,ENSG00000184678,ENSG00000185885,ENSG00000121594,ENSG00000104518,ENSG00000188389,ENSG00000064201,ENSG00000132274,ENSG00000140464,ENSG00000108950,ENSG00000100368,ENSG00000116514,ENSG00000173821,ENSG00000166710,ENSG00000108700,ENSG00000128383,ENSG00000125730,ENSG00000162739,ENSG00000239713,ENSG00000132514,ENSG00000186074,ENSG00000103313,ENSG00000161929,ENSG00000119917,ENSG00000174123,ENSG00000163874,ENSG00000163131,ENSG00000167914,ENSG00000105639,ENSG00000083799,ENSG00000119922,ENSG00000196954,ENSG00000035720,ENSG00000124201,ENSG00000139832,ENSG00000185507,ENSG00000204397,ENSG00000185880,ENSG00000165806,ENSG00000081985,ENSG00000134321,ENSG00000120436,ENSG00000100985,ENSG00000137959,ENSG00000182326,ENSG00000078401,ENSG00000139178,ENSG00000115267,ENSG00000166920,ENSG00000117090,ENSG00000158517,ENSG00000163840,ENSG00000183347,ENSG00000141682,ENSG00000239998,ENSG00000064932,ENSG00000204616,ENSG00000137628,ENSG00000159403,ENSG00000154639,ENSG00000142089,ENSG00000167992,ENSG00000075240,ENSG00000204632,ENSG00000158714,ENSG00000125355,ENSG00000187554,ENSG00000171522,ENSG00000104856,ENSG00000175175,ENSG00000275385,ENSG00000134326,ENSG00000189067,ENSG00000178999,ENSG00000271503,ENSG00000254087,ENSG00000106952,ENSG00000168811,ENSG00000111335,ENSG00000163735,ENSG00000255833,ENSG00000163564,ENSG00000152778,ENSG00000117632,ENSG00000164300,ENSG00000136044,ENSG00000173193,ENSG00000196664,ENSG00000085265,ENSG00000243414,ENSG00000184371,ENSG00000226979,ENSG00000175445,ENSG00000154920,ENSG00000157873,ENSG00000136560,ENSG00000111331,ENSG00000114698,ENSG00000138496,ENSG00000092445,ENSG00000188313,ENSG00000137265
#> 8181 ENSG00000125347,ENSG00000162645,ENSG00000145365,ENSG00000137496,ENSG00000154451,ENSG00000231389,ENSG00000100342,ENSG00000162654,ENSG00000164509,ENSG00000204267,ENSG00000131203,ENSG00000213886,ENSG00000163568,ENSG00000101017,ENSG00000117228,ENSG00000244731,ENSG00000019582,ENSG00000089041,ENSG00000179583,ENSG00000136436,ENSG00000188820,ENSG00000120217,ENSG00000131979,ENSG00000096996,ENSG00000169248,ENSG00000117226,ENSG00000026751,ENSG00000224389,ENSG00000197646,ENSG00000164136,ENSG00000149131,ENSG00000186439,ENSG00000089692,ENSG00000184588,ENSG00000073861,ENSG00000173369,ENSG00000169245,ENSG00000196126,ENSG00000167207,ENSG00000204642,ENSG00000079385,ENSG00000181374,ENSG00000234745,ENSG00000000971,ENSG00000213809,ENSG00000140853,ENSG00000243649,ENSG00000204592,ENSG00000102794,ENSG00000168062,ENSG00000153064,ENSG00000138755,ENSG00000172183,ENSG00000141655,ENSG00000206503,ENSG00000123609,ENSG00000123240,ENSG00000115415,ENSG00000213512,ENSG00000162931,ENSG00000204525,ENSG00000198736,ENSG00000096968,ENSG00000168329,ENSG00000127951,ENSG00000013374,ENSG00000151651,ENSG00000137193,ENSG00000135148,ENSG00000178726,ENSG00000158270,ENSG00000165949,ENSG00000104312,ENSG00000176485,ENSG00000101916,ENSG00000156587,ENSG00000123685,ENSG00000166278,ENSG00000018280,ENSG00000170581,ENSG00000108688,ENSG00000197272,ENSG00000101412,ENSG00000068079,ENSG00000153898,ENSG00000162692,ENSG00000140465,ENSG00000185201,ENSG00000284690,ENSG00000136514,ENSG00000205846,ENSG00000275718,ENSG00000243811,ENSG00000124256,ENSG00000184678,ENSG00000185885,ENSG00000121594,ENSG00000104518,ENSG00000064201,ENSG00000132274,ENSG00000140464,ENSG00000108950,ENSG00000100368,ENSG00000116514,ENSG00000173821,ENSG00000166710,ENSG00000108700,ENSG00000128383,ENSG00000125730,ENSG00000162739,ENSG00000239713,ENSG00000132514,ENSG00000186074,ENSG00000103313,ENSG00000161929,ENSG00000119917,ENSG00000174123,ENSG00000163874,ENSG00000163131,ENSG00000167914,ENSG00000105639,ENSG00000083799,ENSG00000119922,ENSG00000196954,ENSG00000035720,ENSG00000124201,ENSG00000139832,ENSG00000185507,ENSG00000204397,ENSG00000185880,ENSG00000165806,ENSG00000081985,ENSG00000134321,ENSG00000120436,ENSG00000100985,ENSG00000137959,ENSG00000182326,ENSG00000078401,ENSG00000139178,ENSG00000115267,ENSG00000166920,ENSG00000117090,ENSG00000158517,ENSG00000163840,ENSG00000183347,ENSG00000141682,ENSG00000239998,ENSG00000064932,ENSG00000204616,ENSG00000137628,ENSG00000159403,ENSG00000154639,ENSG00000142089,ENSG00000167992,ENSG00000075240,ENSG00000204632,ENSG00000158714,ENSG00000125355,ENSG00000187554,ENSG00000171522,ENSG00000104856,ENSG00000175175,ENSG00000275385,ENSG00000134326,ENSG00000189067,ENSG00000178999,ENSG00000271503,ENSG00000254087,ENSG00000106952,ENSG00000168811,ENSG00000111335,ENSG00000163735,ENSG00000255833,ENSG00000163564,ENSG00000152778,ENSG00000117632,ENSG00000164300,ENSG00000136044,ENSG00000173193,ENSG00000196664,ENSG00000085265,ENSG00000243414,ENSG00000184371,ENSG00000226979,ENSG00000175445,ENSG00000157873,ENSG00000136560,ENSG00000111331,ENSG00000114698,ENSG00000138496,ENSG00000092445,ENSG00000188313,ENSG00000137265
#> 6262 ENSG00000125347,ENSG00000162645,ENSG00000145365,ENSG00000137496,ENSG00000154451,ENSG00000231389,ENSG00000100342,ENSG00000162654,ENSG00000164509,ENSG00000204267,ENSG00000131203,ENSG00000213886,ENSG00000163568,ENSG00000101017,ENSG00000117228,ENSG00000244731,ENSG00000019582,ENSG00000089041,ENSG00000179583,ENSG00000136436,ENSG00000188820,ENSG00000120217,ENSG00000131979,ENSG00000096996,ENSG00000169248,ENSG00000117226,ENSG00000026751,ENSG00000224389,ENSG00000197646,ENSG00000164136,ENSG00000149131,ENSG00000186439,ENSG00000089692,ENSG00000184588,ENSG00000073861,ENSG00000173369,ENSG00000169245,ENSG00000196126,ENSG00000167207,ENSG00000204642,ENSG00000079385,ENSG00000181374,ENSG00000234745,ENSG00000000971,ENSG00000213809,ENSG00000140853,ENSG00000243649,ENSG00000204592,ENSG00000102794,ENSG00000168062,ENSG00000153064,ENSG00000138755,ENSG00000172183,ENSG00000141655,ENSG00000206503,ENSG00000123609,ENSG00000123240,ENSG00000115415,ENSG00000213512,ENSG00000162931,ENSG00000204525,ENSG00000198736,ENSG00000096968,ENSG00000168329,ENSG00000127951,ENSG00000013374,ENSG00000151651,ENSG00000137193,ENSG00000135148,ENSG00000178726,ENSG00000158270,ENSG00000165949,ENSG00000104312,ENSG00000176485,ENSG00000101916,ENSG00000156587,ENSG00000123685,ENSG00000166278,ENSG00000018280,ENSG00000170581,ENSG00000108688,ENSG00000197272,ENSG00000101412,ENSG00000068079,ENSG00000153898,ENSG00000162692,ENSG00000140465,ENSG00000185201,ENSG00000284690,ENSG00000136514,ENSG00000205846,ENSG00000275718,ENSG00000243811,ENSG00000124256,ENSG00000184678,ENSG00000185885,ENSG00000121594,ENSG00000104518,ENSG00000064201,ENSG00000132274,ENSG00000140464,ENSG00000108950,ENSG00000100368,ENSG00000116514,ENSG00000173821,ENSG00000166710,ENSG00000108700,ENSG00000128383,ENSG00000125730,ENSG00000162739,ENSG00000239713,ENSG00000132514,ENSG00000186074,ENSG00000103313,ENSG00000161929,ENSG00000119917,ENSG00000174123,ENSG00000163874,ENSG00000163131,ENSG00000167914,ENSG00000105639,ENSG00000083799,ENSG00000119922,ENSG00000196954,ENSG00000035720,ENSG00000124201,ENSG00000139832,ENSG00000185507,ENSG00000204397,ENSG00000185880,ENSG00000165806,ENSG00000081985,ENSG00000134321,ENSG00000120436,ENSG00000100985,ENSG00000137959,ENSG00000182326,ENSG00000078401,ENSG00000139178,ENSG00000115267,ENSG00000166920,ENSG00000117090,ENSG00000158517,ENSG00000163840,ENSG00000183347,ENSG00000141682,ENSG00000239998,ENSG00000064932,ENSG00000204616,ENSG00000137628,ENSG00000159403,ENSG00000154639,ENSG00000142089,ENSG00000167992,ENSG00000075240,ENSG00000204632,ENSG00000158714,ENSG00000125355,ENSG00000187554,ENSG00000171522,ENSG00000104856,ENSG00000175175,ENSG00000275385,ENSG00000134326,ENSG00000189067,ENSG00000178999,ENSG00000271503,ENSG00000254087,ENSG00000106952,ENSG00000168811,ENSG00000111335,ENSG00000163735,ENSG00000255833,ENSG00000163564,ENSG00000152778,ENSG00000117632,ENSG00000164300,ENSG00000136044,ENSG00000173193,ENSG00000196664,ENSG00000085265,ENSG00000243414,ENSG00000184371,ENSG00000226979,ENSG00000175445,ENSG00000157873,ENSG00000136560,ENSG00000111331,ENSG00000114698,ENSG00000138496,ENSG00000092445,ENSG00000188313,ENSG00000137265
#> genesymbols
#> 1766 ACOD1,ADAM8,ADGRE5,AIM2,APOBEC3A,APOBEC3D,APOBEC3G,APOL1,APPL2,ASCL2,AURKB,B2M,BANK1,BCL6,BTN2A2,BTN3A1,BTN3A2,BTN3A3,C1QB,C1R,C1RL,C1S,C2,C3,C4A,C4B,CALCOCO2,CALHM6,CASP4,CCL13,CCL15,CCL18,CCL5,CCL7,CCL8,CCRL2,CD1A,CD1C,CD274,CD28,CD300H,CD300LF,CD38,CD40,CD69,CD7,CD74,CD80,CD83,CEACAM1,CFB,CFH,CIITA,CLEC10A,CLEC6A,COLEC12,CR1L,CSF1,CSF2RB,CTLA4,CTSL,CTSS,CX3CR1,CXCL10,CXCL11,CXCL5,CXCL9,CXCR3,CYLD,CYSLTR1,CYSLTR2,DDX60,DLL1,DTX3L,DYSF,EDA,EDN1,ENPP2,ERAP2,EXO1,FAS,FCN1,FGL1,FGL2,FYB1,GBP1,GBP2,GBP3,GBP4,GBP5,GBP6,GBP7,GCH1,GPR183,GPR31,GRAMD4,GSDMD,GTPBP1,H2BC21,HLA-A,HLA-B,HLA-C,HLA-DMA,HLA-DMB,HLA-DOA,HLA-DOB,HLA-DPA1,HLA-DPB1,HLA-DQA1,HLA-DQA1,HLA-DQB1,HLA-DQB2,HLA-DRA,HLA-DRB1,HLA-DRB5,HLA-E,HLA-F,HLA-G,HLA-H,HRH2,ICAM1,IDO1,IFI27,IFI35,IFI44L,IFIH1,IFIT2,IFIT3,IFIT5,IFITM1,IFITM2,IFITM3,IGSF6,IL12A,IL12RB1,IL15,IL18BP,IL1RN,IL27,IL31RA,IL32,IL4I1,IL7,IRF1,IRF4,IRF7,ISG20,ITK,JAK2,JAK3,KARS1,KLRK1,LAG3,LAMP3,LILRA1,LILRA2,LILRB3,LITAF,LTA,LY75,LYN,MCOLN2,MEFV,MSRB1,NCF1,NLRC5,NMI,NOD2,NR4A3,NUB1,OAS2,OAS3,OPTN,OSM,P2RX7,PARP14,PARP3,PARP9,PCYT1A,PDCD1,PDCD1LG2,PDE4B,PIM1,PLPP4,PLSCR1,PML,PSMB10,PTGER4,PYHIN1,RAB20,RELB,RIPK2,RNF19B,RSAD2,S100A13,SBNO2,SCIMP,SECTM1,SERINC5,SERPING1,SIT1,SLAMF1,SLAMF6,SLAMF7,SLAMF8,SLC11A1,STAP1,STAT1,STAT2,SUCNR1,TANK,TAP1,TAP2,TBX21,TICAM2,TIFA,TIFAB,TLR10,TLR5,TLR7,TLR8,TNFRSF11A,TNFRSF14,TNFRSF21,TNFSF10,TNFSF13B,TNFSF18,TNFSF8,TRAFD1,TRIM17,TRIM22,TRIM31,TRIM69,TYRO3,UBD,UBE2L6,UNG,ZBP1,ZC3H12A,ZNFX1
#> 610 ACOD1,ADAM8,ADGRE5,AIM2,ALAS1,APOBEC3A,APOBEC3D,APOBEC3G,APOL1,APPL2,ASB2,ASCL2,AURKB,B2M,BANK1,BATF2,BATF3,BCL2L11,BCL6,BTN2A2,BTN3A1,BTN3A2,BTN3A3,C1QB,C1R,C1RL,C1S,C2,C3,C4A,C4B,CALCOCO2,CALHM6,CASP4,CCL13,CCL15,CCL18,CCL5,CCL7,CCL8,CCRL2,CD101,CD1A,CD1C,CD274,CD28,CD300H,CD300LF,CD38,CD40,CD69,CD7,CD74,CD80,CD83,CD93,CEACAM1,CFB,CFH,CIITA,CLEC10A,CLEC2B,CLEC6A,COLEC12,CR1L,CSF1,CSF2RB,CTLA4,CTSL,CTSS,CX3CR1,CXADR,CXCL10,CXCL11,CXCL5,CXCL9,CXCR3,CYLD,CYSLTR1,CYSLTR2,DDX60,DLL1,DTX3L,DYSF,EDA,EDN1,ENPP2,EPAS1,EPHB3,ERAP2,EXO1,FAS,FAXDC2,FCN1,FGL1,FGL2,FLT3LG,FYB1,GBP1,GBP2,GBP3,GBP4,GBP5,GBP6,GBP7,GCH1,GPR183,GPR31,GRAMD4,GSDMD,GTPBP1,H2BC21,HAVCR1,HCAR2,HELLS,HLA-A,HLA-B,HLA-C,HLA-DMA,HLA-DMB,HLA-DOA,HLA-DOB,HLA-DPA1,HLA-DPB1,HLA-DQA1,HLA-DQA1,HLA-DQB1,HLA-DQB2,HLA-DRA,HLA-DRB1,HLA-DRB5,HLA-E,HLA-F,HLA-G,HLA-H,HRH2,HSD3B7,HSH2D,ICAM1,IDO1,IDO2,IFI27,IFI35,IFI44L,IFIH1,IFIT2,IFIT3,IFIT5,IFITM1,IFITM2,IFITM3,IGSF6,IL12A,IL12RB1,IL15,IL15RA,IL18BP,IL1RN,IL27,IL31RA,IL32,IL4I1,IL7,IRF1,IRF4,IRF7,ISG20,ITGA7,ITK,JAK2,JAK3,KARS1,KITLG,KLRK1,LAG3,LAMP3,LILRA1,LILRA2,LILRB3,LITAF,LRP5,LRRK2,LTA,LY75,LYN,LYVE1,MCOLN2,MEFV,MERTK,MSRB1,MTUS1,NCAPG2,NCF1,NLRC5,NLRP12,NMI,NOD2,NR4A3,NUB1,OAS2,OAS3,OPTN,OSM,P2RX4,P2RX7,PADI2,PARP14,PARP3,PARP9,PCYT1A,PDCD1,PDCD1LG2,PDE4B,PIM1,PLPP4,PLSCR1,PMAIP1,PML,POLB,PSMB10,PSMB8,PSMB9,PSME1,PTGER4,PYHIN1,RAB20,RELB,RIPK2,RNF19B,RNF213,RSAD2,S100A13,S1PR1,SBNO2,SCIMP,SCIN,SECTM1,SELL,SERINC5,SERPING1,SIT1,SLAMF1,SLAMF6,SLAMF7,SLAMF8,SLC11A1,SOCS1,STAP1,STAT1,STAT2,SUCNR1,TAL1,TANK,TAP1,TAP2,TAPBP,TAPBPL,TBX21,TESC,TICAM2,TIFA,TIFAB,TLR10,TLR5,TLR7,TLR8,TMEM176A,TMEM176B,TNFAIP6,TNFRSF11A,TNFRSF14,TNFRSF21,TNFSF10,TNFSF13B,TNFSF18,TNFSF8,TRAFD1,TRIM17,TRIM22,TRIM31,TRIM69,TSPAN32,TYRO3,UBD,UBE2L6,UNG,VCAM1,VEGFC,ZBP1,ZC3H12A,ZNFX1
#> 523 ASCL2,B2M,BCL6,BTN3A1,BTN3A2,BTN3A3,C1QB,C1R,C1RL,C1S,C2,C3,C4A,C4B,CD1A,CD1C,CD274,CD28,CD40,CD69,CD7,CD74,CD80,CEACAM1,CLEC10A,CLEC6A,CR1L,CSF2RB,CTLA4,CTSL,CTSS,CX3CR1,ERAP2,EXO1,FGL1,GPR183,HLA-A,HLA-B,HLA-C,HLA-DMA,HLA-DMB,HLA-DOA,HLA-DOB,HLA-DPA1,HLA-DPB1,HLA-DQA1,HLA-DQA1,HLA-DQB1,HLA-DQB2,HLA-DRA,HLA-DRB1,HLA-DRB5,HLA-E,HLA-F,HLA-G,HLA-H,ICAM1,IL12A,IL12RB1,IL18BP,IL27,IL4I1,IRF1,IRF4,IRF7,ITK,JAK2,JAK3,KLRK1,LAG3,LAMP3,LILRA1,LILRB3,LTA,LYN,MCOLN2,NOD2,P2RX7,PARP3,PCYT1A,PDCD1,PDCD1LG2,RELB,RIPK2,RNF19B,RSAD2,SECTM1,SERPING1,SIT1,SLAMF1,SLAMF6,SLAMF7,SLC11A1,TAP1,TAP2,TBX21,TLR8,TNFRSF11A,TNFRSF14,TNFRSF21,TNFSF13B,TNFSF18,UNG,ZC3H12A
#> 2392 ACOD1,ADAM8,AIM2,APOBEC3A,APOBEC3D,APOBEC3G,APOL1,APPL2,AURKB,B2M,BANK1,BATF2,BATF3,C15orf48,C1QB,C1R,C1RL,C1S,C2,C3,C4A,C4B,CALCOCO2,CALHM6,CARD16,CASP4,CASP7,CCL13,CCL15,CCL18,CCL5,CCL7,CCL8,CD274,CD300H,CD300LF,CD40,CD74,CD80,CEACAM1,CFB,CFH,CIITA,CLEC10A,CLEC6A,CMPK2,COLEC12,CSF1,CSF2RB,CTSS,CX3CR1,CXADR,CXCL10,CXCL11,CXCL5,CXCL9,CYLD,CYP1A1,DDX60,DTX3L,E2F1,EDN1,EME1,FAM20A,FCN1,FGL2,GBP1,GBP2,GBP3,GBP4,GBP5,GBP6,GBP7,GCH1,GPR31,GRAMD4,GSDMA,GSDMD,H2BC21,HLA-A,HLA-B,HLA-C,HLA-DPA1,HLA-DRB1,HLA-E,HLA-F,HLA-G,IDO1,IFI27,IFI35,IFI44L,IFIH1,IFIT2,IFIT3,IFIT5,IFITM1,IFITM2,IFITM3,IL12A,IL12RB1,IL12RB2,IL15,IL18BP,IL27,IL31RA,IL4I1,IRF1,IRF4,IRF7,ISG20,JAK2,JAK3,KLF4,KLRK1,LAG3,LILRA2,LITAF,LPL,LTA,LYN,MCOLN2,MEFV,MMP9,MSRB1,NCF1,NLRC5,NMI,NOD2,NUB1,OAS2,OAS3,OPTN,P2RX7,PARP14,PARP9,PDCD1,PDCD1LG2,PDE4B,PIM1,PLAAT3,PLSCR1,PLSCR4,PMAIP1,PML,PPM1E,PTGER4,PYHIN1,RAB20,RELB,RIPK2,RNF19B,RNF213,RSAD2,RTP4,SBNO2,SCIMP,SERINC5,SERPING1,SLAMF1,SLAMF6,SLAMF7,SLAMF8,SLC11A1,STAP1,STAT1,STAT2,STMN1,TANK,TAP2,TBX21,THBD,TICAM2,TIFA,TIFAB,TLR10,TLR5,TLR7,TLR8,TMEM255A,TNFRSF11A,TNFRSF14,TNFSF8,TRAFD1,TRDN,TRIM17,TRIM22,TRIM31,TRIM69,TSPAN32,TYRO3,UBD,UBE2L6,VCAM1,VWCE,ZBP1,ZC3H12A,ZNFX1
#> 8181 ACOD1,ADAM8,AIM2,APOBEC3A,APOBEC3D,APOBEC3G,APOL1,APPL2,AURKB,B2M,BANK1,BATF2,BATF3,C15orf48,C1QB,C1R,C1RL,C1S,C2,C3,C4A,C4B,CALCOCO2,CALHM6,CARD16,CASP4,CASP7,CCL13,CCL15,CCL18,CCL5,CCL7,CCL8,CD274,CD300H,CD300LF,CD40,CD74,CD80,CEACAM1,CFB,CFH,CIITA,CLEC10A,CLEC6A,CMPK2,COLEC12,CSF1,CSF2RB,CTSS,CX3CR1,CXADR,CXCL10,CXCL11,CXCL5,CXCL9,CYLD,CYP1A1,DDX60,DTX3L,E2F1,EDN1,FAM20A,FCN1,FGL2,GBP1,GBP2,GBP3,GBP4,GBP5,GBP6,GBP7,GCH1,GPR31,GRAMD4,GSDMA,GSDMD,H2BC21,HLA-A,HLA-B,HLA-C,HLA-DPA1,HLA-DRB1,HLA-E,HLA-F,HLA-G,IDO1,IFI27,IFI35,IFI44L,IFIH1,IFIT2,IFIT3,IFIT5,IFITM1,IFITM2,IFITM3,IL12A,IL12RB1,IL12RB2,IL15,IL18BP,IL27,IL31RA,IRF1,IRF4,IRF7,ISG20,JAK2,JAK3,KLRK1,LAG3,LILRA2,LITAF,LPL,LTA,LYN,MCOLN2,MEFV,MMP9,MSRB1,NCF1,NLRC5,NMI,NOD2,NUB1,OAS2,OAS3,OPTN,P2RX7,PARP14,PARP9,PDCD1LG2,PDE4B,PIM1,PLAAT3,PLSCR1,PLSCR4,PMAIP1,PML,PPM1E,PTGER4,PYHIN1,RAB20,RELB,RIPK2,RNF19B,RNF213,RSAD2,RTP4,SBNO2,SCIMP,SERINC5,SERPING1,SLAMF1,SLAMF6,SLAMF7,SLAMF8,SLC11A1,STAP1,STAT1,STAT2,STMN1,TANK,TAP2,TBX21,THBD,TICAM2,TIFA,TIFAB,TLR10,TLR5,TLR7,TLR8,TMEM255A,TNFRSF11A,TNFRSF14,TNFSF8,TRAFD1,TRDN,TRIM17,TRIM22,TRIM31,TRIM69,TSPAN32,TYRO3,UBD,UBE2L6,VCAM1,VWCE,ZBP1,ZC3H12A,ZNFX1
#> 6262 ACOD1,ADAM8,AIM2,APOBEC3A,APOBEC3D,APOBEC3G,APOL1,APPL2,AURKB,B2M,BANK1,BATF2,BATF3,C15orf48,C1QB,C1R,C1RL,C1S,C2,C3,C4A,C4B,CALCOCO2,CALHM6,CARD16,CASP4,CASP7,CCL13,CCL15,CCL18,CCL5,CCL7,CCL8,CD274,CD300H,CD300LF,CD40,CD74,CD80,CEACAM1,CFB,CFH,CIITA,CLEC10A,CLEC6A,CMPK2,COLEC12,CSF1,CSF2RB,CTSS,CX3CR1,CXADR,CXCL10,CXCL11,CXCL5,CXCL9,CYLD,CYP1A1,DDX60,DTX3L,E2F1,EDN1,FAM20A,FCN1,FGL2,GBP1,GBP2,GBP3,GBP4,GBP5,GBP6,GBP7,GCH1,GPR31,GRAMD4,GSDMA,GSDMD,H2BC21,HLA-A,HLA-B,HLA-C,HLA-DPA1,HLA-DRB1,HLA-E,HLA-F,HLA-G,IDO1,IFI27,IFI35,IFI44L,IFIH1,IFIT2,IFIT3,IFIT5,IFITM1,IFITM2,IFITM3,IL12A,IL12RB1,IL12RB2,IL15,IL18BP,IL27,IL31RA,IRF1,IRF4,IRF7,ISG20,JAK2,JAK3,KLRK1,LAG3,LILRA2,LITAF,LPL,LTA,LYN,MCOLN2,MEFV,MMP9,MSRB1,NCF1,NLRC5,NMI,NOD2,NUB1,OAS2,OAS3,OPTN,P2RX7,PARP14,PARP9,PDCD1LG2,PDE4B,PIM1,PLAAT3,PLSCR1,PLSCR4,PMAIP1,PML,PPM1E,PTGER4,PYHIN1,RAB20,RELB,RIPK2,RNF19B,RNF213,RSAD2,RTP4,SBNO2,SCIMP,SERINC5,SERPING1,SLAMF1,SLAMF6,SLAMF7,SLAMF8,SLC11A1,STAP1,STAT1,STAT2,STMN1,TANK,TAP2,TBX21,THBD,TICAM2,TIFA,TIFAB,TLR10,TLR5,TLR7,TLR8,TMEM255A,TNFRSF11A,TNFRSF14,TNFSF8,TRAFD1,TRDN,TRIM17,TRIM22,TRIM31,TRIM69,TSPAN32,TYRO3,UBD,UBE2L6,VCAM1,VWCE,ZBP1,ZC3H12A,ZNFX1The function will return a table with 10 columns
-
category: The computer readable GO term ID. -
over_represented_pvalue: p-value for the overrepresentation test for enrichment (as specified by thegoseqalgorithm) -
under_represented_pvalue: p-value for the underrepresentation test for enrichment -
numDEInCat: The number of DE genes associated with this term (as defined in theres_deor in thede_genesparameters) (similar to theAnnotatedcolumn of the output fromrun_topGO()) -
numInCatThe overall number of detected genes associated with this term (as defined in thede_containeror in thebg_genesparameters). -
term: The human readable GO term name. -
ontology: The ontology used to run the enrichment tests (BP/MF/CC) -
p.adj: FDR of your findings adjusted for multiple testing -
genes: Lists the DE gene identifiers associated with that term (separated by a comma) -
genesymbols: Lists the DE gene symbols associated with that term (separated by a comma)
mosdef and clusterProfiler
Parameters like top_de, min_counts,
verbose, FDR_threshold and
de_type can also be used here (For more detail on these
parameters see above). If you want to further customize the call of
enrichGO() inside the function, have a look at the
documentation for enrichGO() from clusterProfiler
Those parameters can be added to the run_cluPro() function
call within the ellipsis (...). For example, as we are
doing in the chunk that follows, we set Biological Process as the
ontology to be used, by specifying ont = "BP"
clupro_macrophage <- run_cluPro(
de_container = dds_macrophage,
res_de = res_macrophage_IFNg_vs_naive,
mapping = "org.Hs.eg.db",
keyType = "SYMBOL",
ont = "BP"
)
head(clupro_macrophage)Importantly, keyType is relevant for the
enrichGO() function that is wrapped in this routine. If
using DESeqDataset and DESeqResults, this has
to be “SYMBOL” which is also the default. If you use vectors please
specify here what type of IDs you provide.
Again, to save time when rendering the vignette, we load the objects provided alongside this package to demonstrate the output (see also this script included in the package to inspect the code used to generate the objects):
data(res_enrich_macrophage_cluPro, package = "mosdef")The function will return a large enrich result containing some metadata and the enrichment results with 9 columns.
head(res_enrich_macrophage_cluPro)
#> ID
#> GO:0002250 GO:0002250
#> GO:0002252 GO:0002252
#> GO:0009617 GO:0009617
#> GO:0002460 GO:0002460
#> GO:0019882 GO:0019882
#> GO:0002396 GO:0002396
#> Description
#> GO:0002250 adaptive immune response
#> GO:0002252 immune effector process
#> GO:0009617 response to bacterium
#> GO:0002460 adaptive immune response based on somatic recombination of immune receptors built from immunoglobulin superfamily domains
#> GO:0019882 antigen processing and presentation
#> GO:0002396 MHC protein complex assembly
#> GeneRatio BgRatio pvalue p.adjust qvalue
#> GO:0002250 99/789 374/13083 4.458881e-38 2.055544e-34 1.713618e-34
#> GO:0002252 90/789 488/13083 2.959548e-22 6.821758e-19 5.687005e-19
#> GO:0009617 86/789 485/13083 3.940453e-20 6.055163e-17 5.047928e-17
#> GO:0002460 60/789 261/13083 6.133599e-20 7.068973e-17 5.893098e-17
#> GO:0019882 36/789 103/13083 8.753721e-19 8.070930e-16 6.728386e-16
#> GO:0002396 16/789 18/13083 3.616983e-18 2.144588e-15 1.787850e-15
#> geneID
#> GO:0002250 IRF1/IL18BP/HLA-DPA1/HLA-DMA/TAP2/TAP1/HLA-DMB/ASCL2/CD40/C4A/CD74/HLA-DOA/P2RX7/CD274/HLA-DRA/IL12RB1/ITK/SLAMF7/C4B/PDCD1LG2/SERPING1/LAG3/LAMP3/HLA-DPB1/TBX21/ERAP2/C1QB/HLA-DRB1/NOD2/HLA-F/CEACAM1/HLA-DQB1/BTN3A2/HLA-DRB5/HLA-B/KLRK1/BTN3A1/HLA-E/TNFRSF11A/HLA-A/CTLA4/HLA-C/JAK2/CX3CR1/HLA-DQB2/CR1L/CD7/RIPK2/TLR8/BTN3A3/IL4I1/C2/TNFSF13B/SLC11A1/GPR183/IL27/CD28/MCOLN2/HLA-DOB/HLA-DQA1/ICAM1/CLEC6A/LILRB3/TNFSF18/CD80/PDCD1/CSF2RB/TNFRSF21/RNF19B/B2M/C3/SLAMF6/CLEC10A/ZC3H12A/CTSS/JAK3/FGL1/CD1A/EXO1/IRF7/RSAD2/LILRA1/C1S/C1RL/SIT1/SLAMF1/C1R/HLA-G/CD1C/RELB/BCL6/LYN/IL12A/UNG/LTA/TNFRSF14/PARP3/CTSL/IRF4
#> GO:0002252 TAP2/HLA-DMB/ASCL2/CD40/C4A/CD74/P2RX7/HLA-DRA/IL12RB1/SLAMF7/C4B/SERPING1/LAG3/TBX21/C1QB/HLA-DRB1/NOD2/HLA-F/CEACAM1/BTN3A2/HLA-B/CFH/KLRK1/CFB/HLA-E/HLA-A/NMI/HLA-C/SUCNR1/CX3CR1/FGL2/CR1L/RIPK2/TLR8/BTN3A3/IL4I1/C2/SLC11A1/GPR183/IL27/IFI35/CD28/ICAM1/TNFSF18/CD80/CSF2RB/RNF19B/B2M/C3/SLAMF6/SCIMP/S100A13/ZC3H12A/JAK3/PLPP4/STAP1/CD1A/EXO1/IRF7/RSAD2/C1S/C1RL/SLAMF1/NCF1/LILRA2/SBNO2/DDX60/DYSF/C1R/KARS1/HLA-G/SLAMF8/CD1C/PTGER4/RELB/BCL6/LITAF/LYN/IL12A/APPL2/DLL1/TLR7/FCN1/TICAM2/UNG/LTA/NR4A3/TNFRSF14/PARP3/IRF4
#> GO:0009617 GBP2/IL18BP/GBP5/GBP4/TAP2/IDO1/CD40/GBP1/P2RX7/CD274/GCH1/CXCL11/GBP3/C4B/PDCD1LG2/TRDN/PDE4B/CXCL10/HLA-DRB1/NOD2/HLA-B/KLRK1/NLRC5/CFB/HLA-E/ACOD1/BANK1/CXCL9/TNFRSF11A/HLA-A/OPTN/GBP7/JAK2/CX3CR1/THBD/COLEC12/RIPK2/PLAAT3/C2/SLC11A1/IL27/VCAM1/CYP1A1/H2BC21/CD80/GSDMD/FAM20A/CSF2RB/RNF213/B2M/C3/SCIMP/TLR10/ZC3H12A/GSDMA/CASP4/STAP1/ZNFX1/CARD16/CASP7/IL12RB2/GPR31/MMP9/EDN1/C15orf48/GBP6/LILRA2/SBNO2/SLAMF8/TMEM255A/PTGER4/PPM1E/CMPK2/LITAF/LYN/TNFSF8/IL12A/OAS2/CXCL5/TIFAB/TICAM2/LTA/LPL/TNFRSF14/OAS3/PLSCR4
#> GO:0002460 IL18BP/TAP2/ASCL2/CD40/C4A/CD74/P2RX7/CD274/HLA-DRA/IL12RB1/C4B/SERPING1/TBX21/C1QB/HLA-DRB1/NOD2/HLA-F/CEACAM1/BTN3A2/HLA-B/HLA-E/HLA-A/HLA-C/JAK2/CR1L/RIPK2/TLR8/BTN3A3/IL4I1/C2/TNFSF13B/SLC11A1/IL27/CD28/ICAM1/CLEC6A/CD80/CSF2RB/B2M/C3/SLAMF6/ZC3H12A/JAK3/CD1A/EXO1/IRF7/RSAD2/C1S/C1RL/SLAMF1/C1R/HLA-G/CD1C/RELB/BCL6/IL12A/UNG/LTA/PARP3/IRF4
#> GO:0019882 HLA-DPA1/HLA-DMA/TAP2/TAP1/HLA-DMB/CD74/HLA-DOA/HLA-DRA/HLA-DPB1/ERAP2/HLA-DRB1/NOD2/HLA-F/HLA-DQB1/HLA-DRB5/HLA-B/PSME1/HLA-E/HLA-A/HLA-C/FGL2/HLA-DQB2/SLC11A1/TAPBP/PSMB8/HLA-DOB/HLA-DQA1/ICAM1/B2M/CTSS/CD1A/TAPBPL/HLA-G/CD1C/RELB/CTSL
#> GO:0002396 HLA-DPA1/HLA-DMA/HLA-DMB/HLA-DOA/HLA-DRA/HLA-DPB1/HLA-DRB1/HLA-DQB1/HLA-DRB5/HLA-A/HLA-DQB2/TAPBP/HLA-DOB/HLA-DQA1/B2M/TAPBPL
#> Count
#> GO:0002250 99
#> GO:0002252 90
#> GO:0009617 86
#> GO:0002460 60
#> GO:0019882 36
#> GO:0002396 16The definitions of these columns are included in the extensive clusterProfiler package documentation, please refer to that for more details.
Alternative ways to run enrichment analyses, within
mosdef
All of these functions tailored to run enrichment methods also work
if you only have/provide a vector of differentially expressed genes and
a vector of background genes. Most of the above mentioned parameters
work here as well (top_de, verbose), however
parameters like min_counts and de_type will
not affect the result, since they need further information which can
only be found in the DESeqDataset and
DESeqResults (in this case, access to the counts from the
DESeqDataset object de_container and the
Log2FoldChange from the DESeqResults object passed to
res_de).
res_subset <- deresult_to_df(res_macrophage_IFNg_vs_naive)[1:100, ]
myde <- res_subset$id
myassayed <- rownames(res_macrophage_IFNg_vs_naive)
## Here keys are Ensembl not symbols
res_enrich_macrophage_topGO_vec <- run_topGO(
de_genes = myde,
bg_genes = myassayed,
mapping = "org.Hs.eg.db",
gene_id = "ensembl"
)
#> Your dataset has 100 DE genes.
#> You selected 100 (100.00%) genes for the enrichment analysis.
#> 6075 GO terms were analyzed. Not all of them are significantly enriched.
#> We suggest further subsetting the output list by for example:
#> using a pvalue cutoff in the column:
#> 'p.value_elim'.
head(res_enrich_macrophage_topGO_vec)
#> GO.ID
#> 1 GO:0002503
#> 2 GO:0002250
#> 3 GO:0019886
#> 4 GO:0034341
#> 5 GO:0031640
#> 6 GO:0071346
#> Term
#> 1 peptide antigen assembly with MHC class II protein complex
#> 2 adaptive immune response
#> 3 antigen processing and presentation of exogenous peptide antigen via MHC class II
#> 4 response to type II interferon
#> 5 killing of cells of another organism
#> 6 cellular response to type II interferon
#> Annotated Significant Expected Rank in p.value_classic p.value_elim
#> 1 14 7 0.08 39 7.6e-13
#> 2 385 29 2.33 3 3.2e-12
#> 3 28 8 0.17 43 3.6e-12
#> 4 111 14 0.67 17 8.0e-10
#> 5 50 7 0.30 90 1.9e-08
#> 6 91 8 0.55 104 7.2e-08
#> p.value_classic
#> 1 7.6e-13
#> 2 1.3e-24
#> 3 3.6e-12
#> 4 3.6e-15
#> 5 1.9e-08
#> 6 7.2e-08
#> genes
#> 1 ENSG00000196126,ENSG00000204252,ENSG00000204257,ENSG00000204287,ENSG00000223865,ENSG00000231389,ENSG00000242574
#> 2 ENSG00000019582,ENSG00000026751,ENSG00000073861,ENSG00000078081,ENSG00000089041,ENSG00000089692,ENSG00000096996,ENSG00000101017,ENSG00000113263,ENSG00000120217,ENSG00000125347,ENSG00000137496,ENSG00000149131,ENSG00000164308,ENSG00000167207,ENSG00000168394,ENSG00000173369,ENSG00000183734,ENSG00000196126,ENSG00000197646,ENSG00000204252,ENSG00000204257,ENSG00000204267,ENSG00000204287,ENSG00000223865,ENSG00000224389,ENSG00000231389,ENSG00000242574,ENSG00000244731
#> 3 ENSG00000019582,ENSG00000196126,ENSG00000204252,ENSG00000204257,ENSG00000204287,ENSG00000223865,ENSG00000231389,ENSG00000242574
#> 4 ENSG00000019582,ENSG00000096996,ENSG00000101017,ENSG00000117226,ENSG00000117228,ENSG00000125347,ENSG00000131979,ENSG00000136436,ENSG00000154451,ENSG00000162645,ENSG00000162654,ENSG00000179583,ENSG00000213886,ENSG00000231389
#> 5 ENSG00000100342,ENSG00000117226,ENSG00000117228,ENSG00000154451,ENSG00000162645,ENSG00000169245,ENSG00000169248
#> 6 ENSG00000096996,ENSG00000117226,ENSG00000117228,ENSG00000125347,ENSG00000154451,ENSG00000162645,ENSG00000162654,ENSG00000231389Plotting expression values in the context of DE
mosdef provides some wrappers to commonly used visualizations of individual genes, as well as summary visualizations for all features at once.
Individual genes - gene_plot()
An elegant way to plot the expression values (by default the
normalized counts) of a certain gene of interest, split up by a
covariate of interest - for example, the condition, IFNg vs
naive.
gene_plot(
de_container = dds_macrophage,
gene = "ENSG00000125347",
intgroup = "condition"
)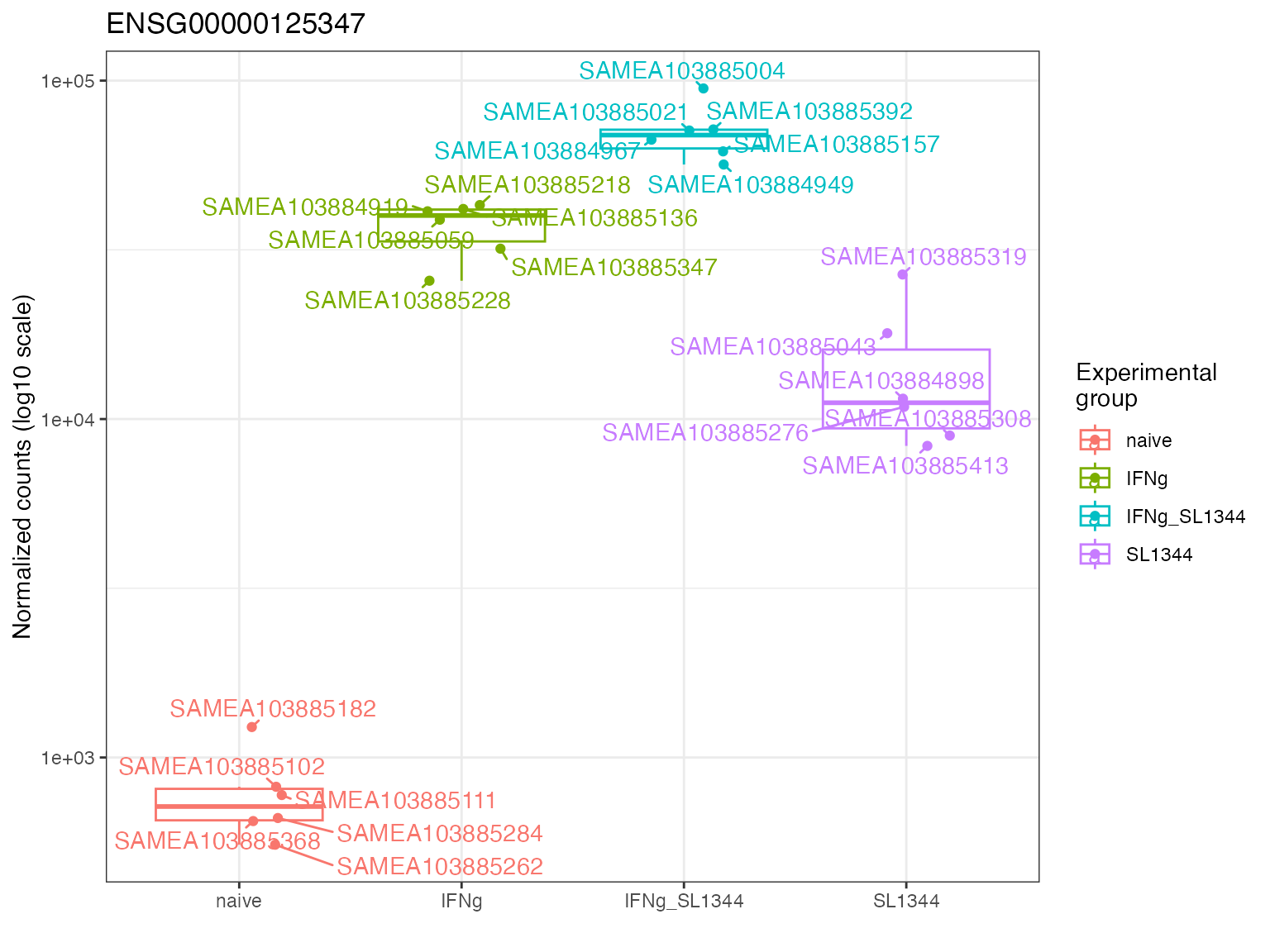
Key parameters are in this case:
-
de_container: YourDESeqDataset -
gene: The gene of interest -
intgroup: A character vector of names incolData(de_container)used for grouping the expression values.
Notably, gene_plot() also has some heuristics to suggest
an appropriate layer of plotting the data points, depending on the
number of samples included in each individual group - this include the
simple jittered points, a boxplot, a violin plot, or a sina plot. This
automatic behavior can be suppressed by specifying a different value for
the plot_type parameter.
All genes at once - Volcano plots
Volcano plots are one of the most well known and used plots to
display differentially expressed genes. These functions return a basic
ggplot object including the most important parts when
creating a volcano plot.
volcPlot <- de_volcano(
res_de = res_macrophage_IFNg_vs_naive,
mapping = "org.Hs.eg.db",
logfc_cutoff = 1,
FDR = 0.05,
labeled_genes = 25
)
#> 'select()' returned 1:many mapping between keys and columns
volcPlot
#> Warning: Removed 17782 rows containing missing values or values outside the scale range
#> (`geom_text_repel()`).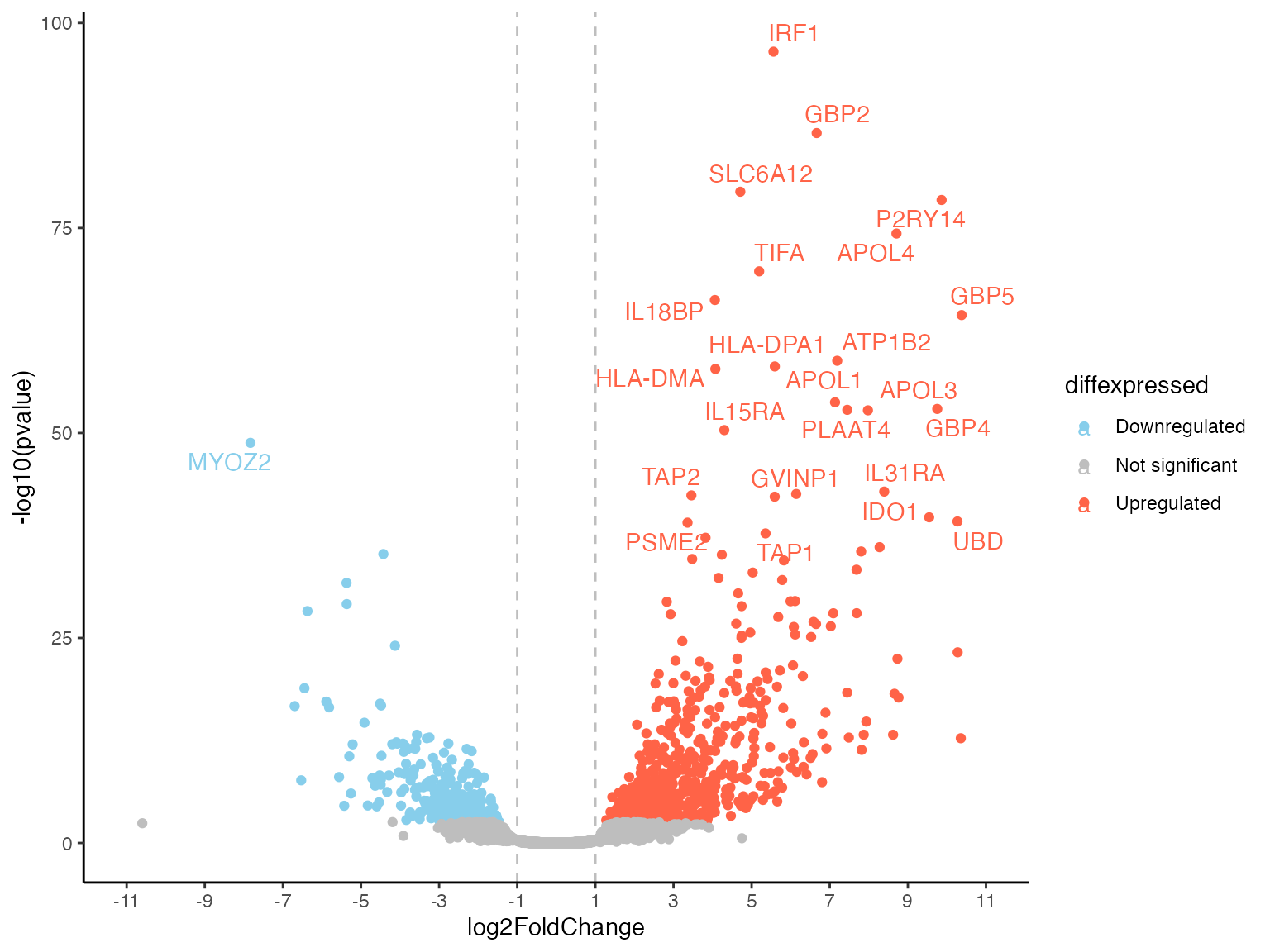
Again, an overview on the key parameters:
-
res_de: YourDESeqResults -
mapping: The annotation/species: Important to generate symbols for labeling. -
labeled_genes: The number of the top DE genes to be labeled. Default is 30. -
logfc_cutoffandFDR: Where to draw the lines in the plot and which genes to mark as significant. The default is 1 (meaning L2FC +/-1): So genes with a FoldChange higher than 1 or lower -1 and a padj value below 0.05.
This plot can be later modified by the user, like any regular
ggplot object. For example:
library("ggplot2")
volcPlot +
ggtitle("macrophage Volcano") +
ylab("-log10 PValue") +
xlab("Log 2 FoldChange (Cutoff 1/-1)")
#> Warning: Removed 17782 rows containing missing values or values outside the scale range
#> (`geom_text_repel()`).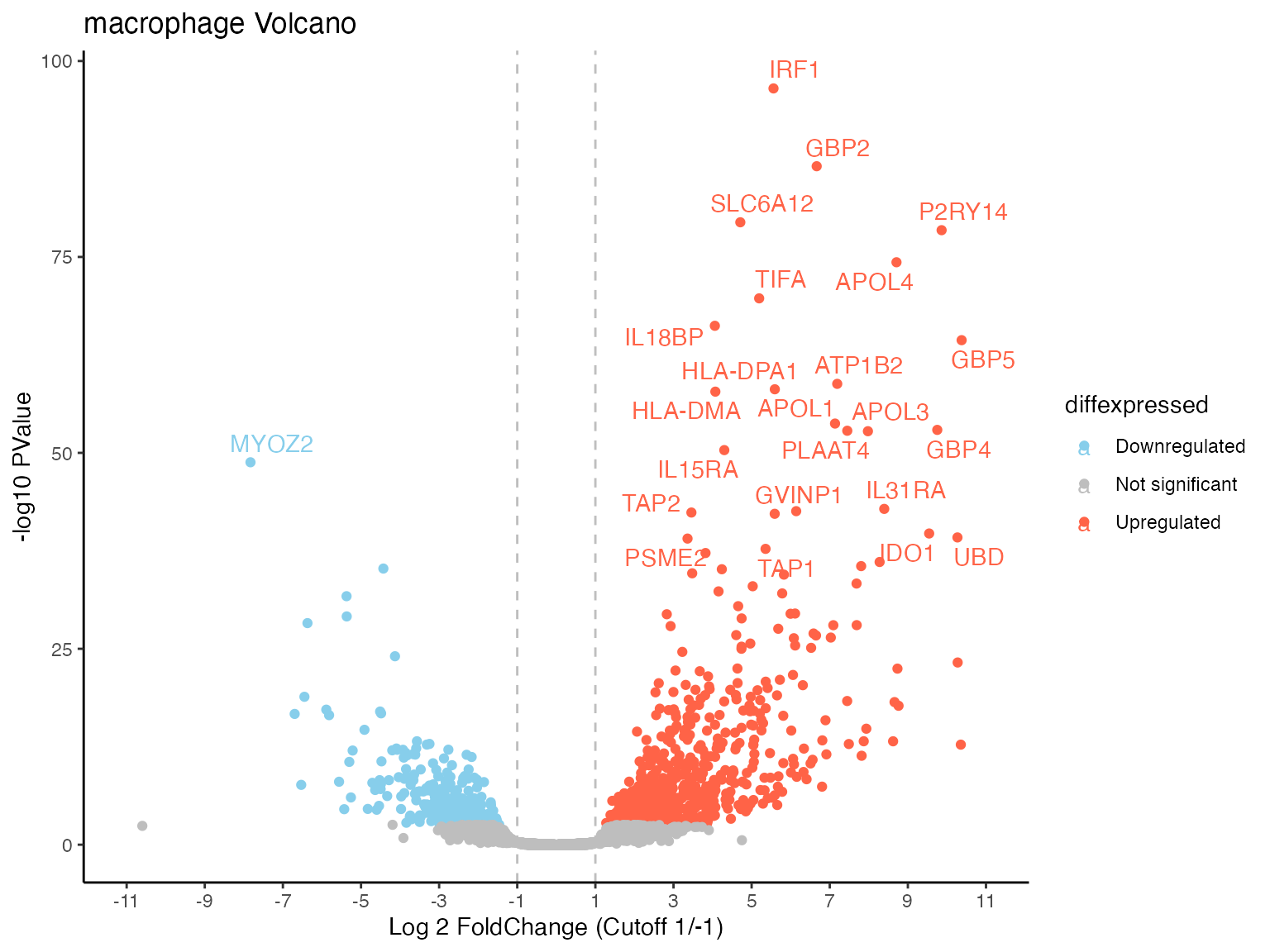
For further possibilities please look at the ggplot2 documentation.
In addition to only focusing on differentially expressed genes, in a
volcano plot the user can also highlight genes associated with a certain
GO term of interest. This can be done with the go_volcano()
function:
Volc_GO <- go_volcano(
res_de = res_macrophage_IFNg_vs_naive,
res_enrich = res_enrich_macrophage_topGO,
term_index = 1,
logfc_cutoff = 1,
FDR = 0.05,
mapping = "org.Hs.eg.db",
n_overlaps = 50,
col_to_use = "symbol",
enrich_col = "genes",
down_col = "black",
up_col = "black",
highlight_col = "tomato"
)
Volc_GO
#> Warning: Removed 17716 rows containing missing values or values outside the scale range
#> (`geom_label_repel()`).
#> Warning: ggrepel: 23 unlabeled data points (too many overlaps). Consider
#> increasing max.overlaps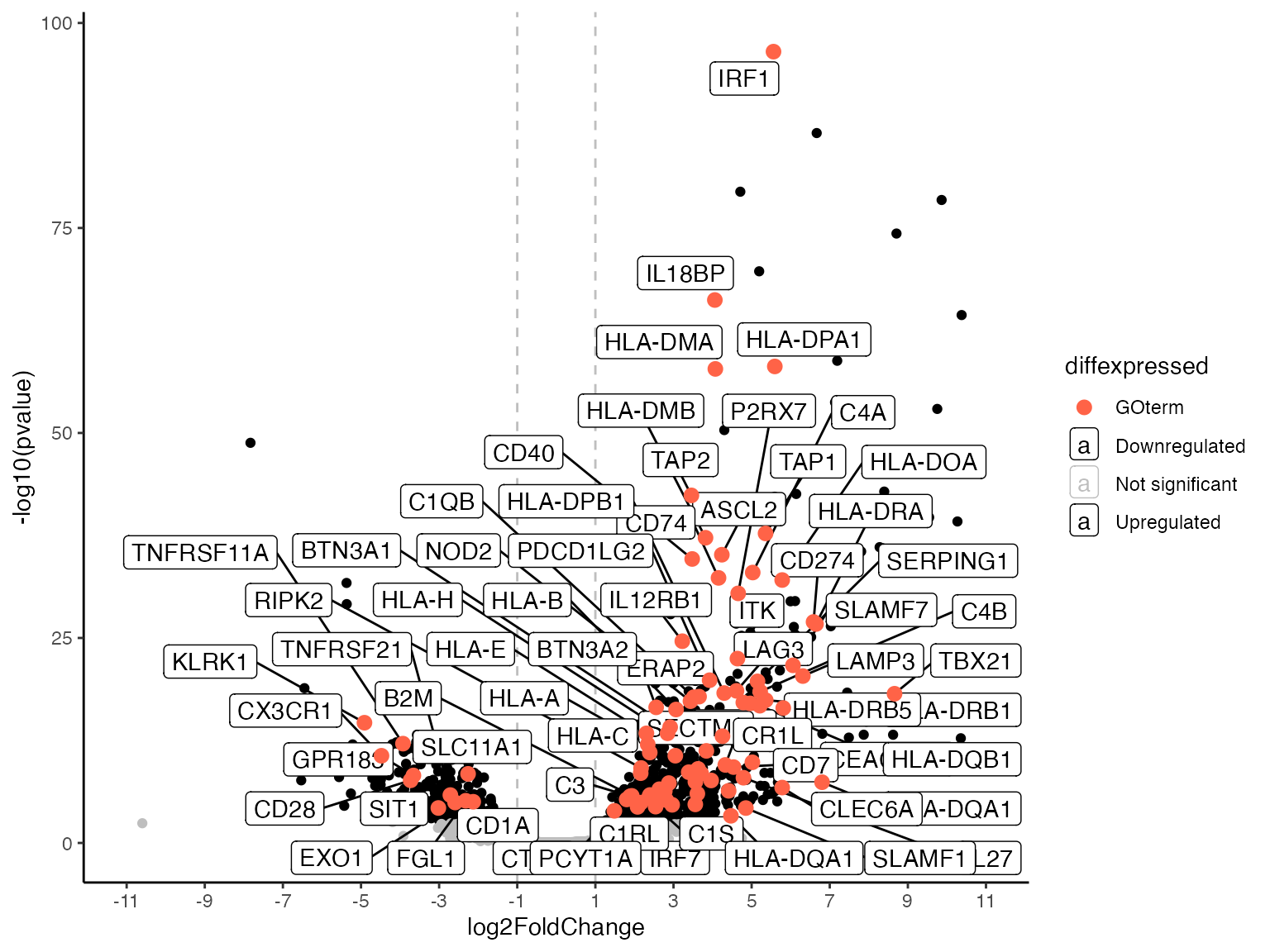
The key parameters:
-
res_de: YourDESeqResults -
res_enrich: Your enrichment results -
term_index: The index(row) where your term of interest is located in your enrichment result. -
logfc_cutoffandFDR: Where to draw the lines in the plot and which genes to mark as significant. The default is one (meaning L2FC +/-1): So genes with a FoldChange higher than 1 or lower -1 and a padj value below 0.05. -
mapping: The annotation/species: Important to generate symbols for labeling. -
n_overlaps: The number of overlapsggrepelis supposed to allow for labeling (increases number of labeled genes). -
col_to_use: Name of the column in your res_de containing the gene symbols. -
enrich_col: Name of the column in your res_enrich containing the gene symbols. For an example seerun_topGOdata provided in mosdef:data(res_enrich_macrophage_topGO, package = "mosdef"). -
down_col: Colour for your downregulated genes (genes with alogfc_cutoffbelow the value specified). -
up_col: Colour for your upregulated genes (genes with alogfc_cutoffabove the value specified). -
highlight_col: Colour for your genes associated with the given term of interest.
All genes at once - MA plots
An alternative to the volcano plot, less focused on the individual significance values and more focused on the combination of mean expression and changes in expression levels, is the MA plot. It can be considered an extension of the Bland-Altman plot for genomics data. This grants an overview of the differentially expressed genes across the different levels of expression.
plot_ma() also allows you to set x and y labels right
away, but we provide some default values. However, similar to
de_volcano(), these can also be set later on by directly
modifying the returned ggplot object.
maplot <- plot_ma(
res_de = res_macrophage_IFNg_vs_naive,
FDR = 0.05,
hlines = 1
)
# For further parameters please check the function documentation
maplot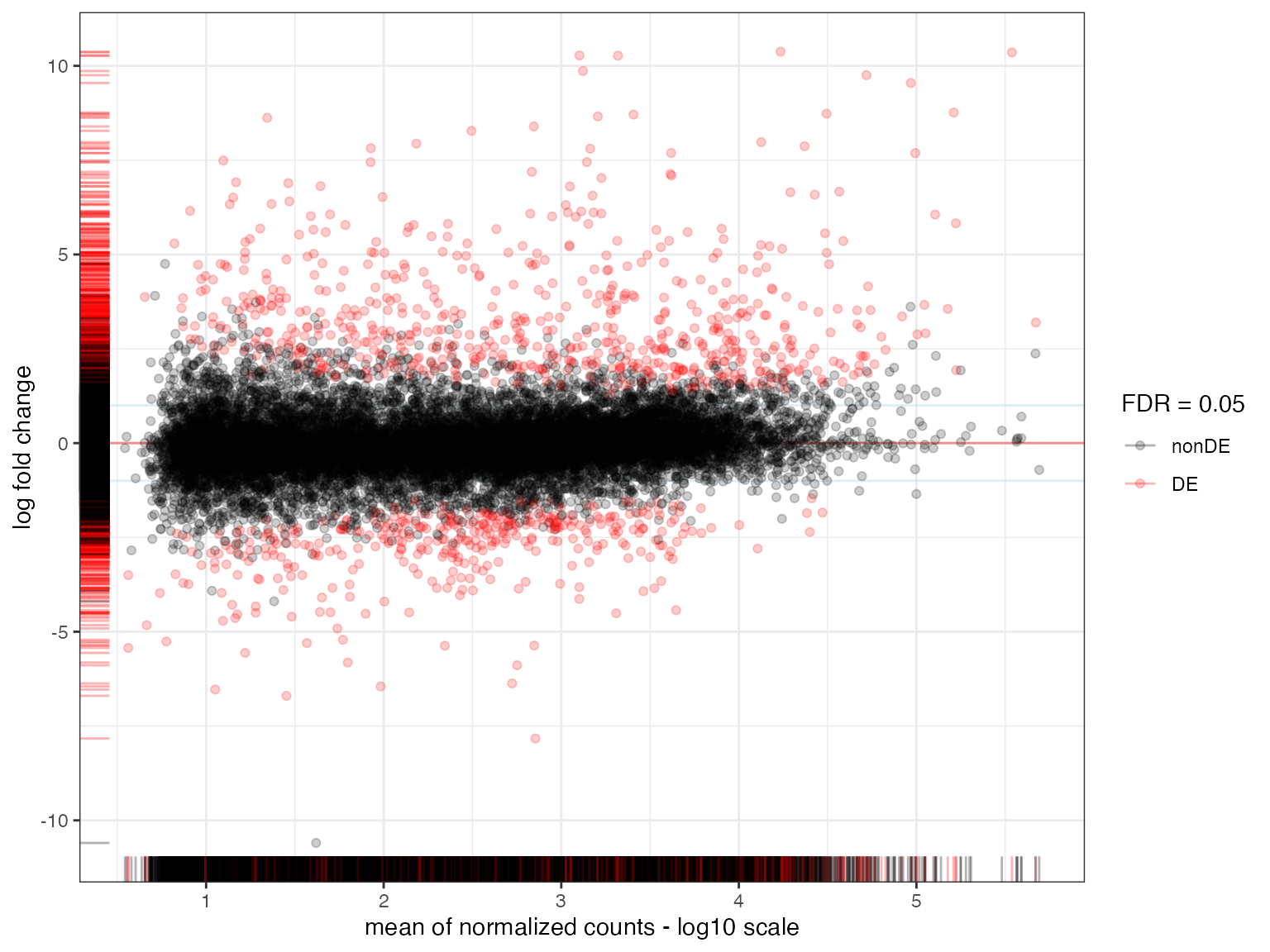
All key parameters at a glance:
-
res_de: YourDESeqResultsobject -
FDR: Which padj cutoff value to set for genes to be counted as DE (default < 0.05) -
hlines: whether or not (and where) to draw the horizontal line (optional)
Further control on the aspect of the output plot is enabled via the
other possible parameters; please refer to the documentation of the
plot_ma() function itself.
If desired, plot_ma() further allows you to highlight
certain genes of interest to you, if providing them via the
intgenes parameter.
maplot_genes <- plot_ma(
res_de = res_macrophage_IFNg_vs_naive,
FDR = 0.1,
add_rug = FALSE,
intgenes = c(
"SLC7A2",
"CFLAR",
"PDK4",
"IFNGR1"
), # suggested genes of interest
hlines = 1,
intgenes_color = "darkblue"
)
maplot_genes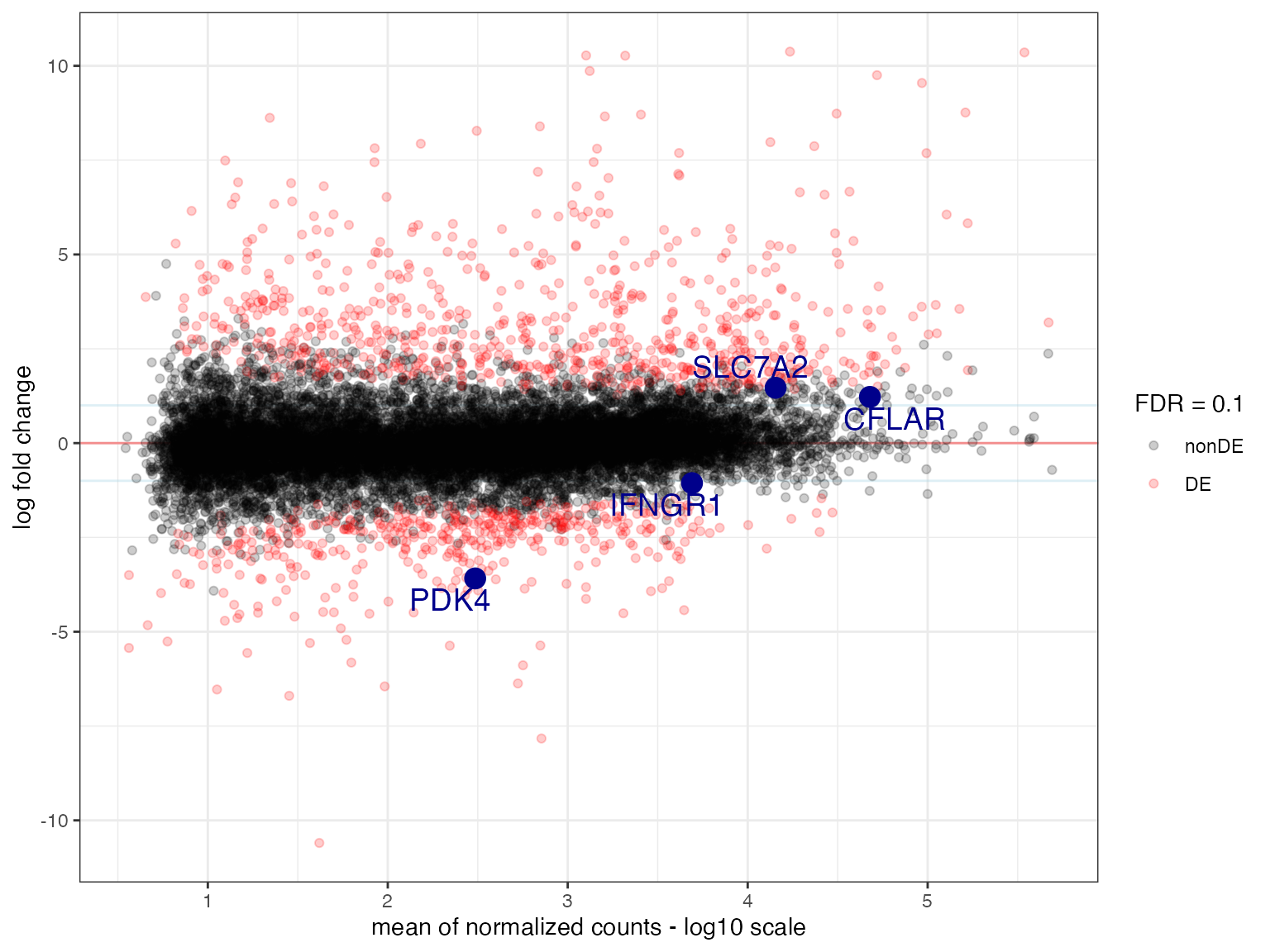
Beautifying and enhancing analysis reports
Analysis reports, often generated via Rmarkdown, are a great way of handing over data, results, and output to collaborators, colleagues, PIs, …
mosdef provides a set of functions aiming to enhance the report quality, e.g. by turning normal tables into interactive tables, linking to a number of additional external databases - thus simplifying the search & exploration steps which naturally follow the inspection of a DE table.
More information on features/genes
The life of a bioinformatician, but also the life of a biologist and a medical scientist, often contains a fair amount of searches into external databases, in order to obtain additional information on the shortlisted features.
Simplifying the time to reach these resources and embedding them into one info-rich analysis report is mosdef’s proposal to streamline this as a whole.
All of these (except ENSEMBL, using their internal identifier system) require gene symbols as the input. Currently, mosdef has functions to create automated links to:
- ENSEMBL (https://www.ensembl.org/index.html)
- GeneCards (https://www.genecards.org/): For information/overview on the gene
- Pubmed (https://pubmed.ncbi.nlm.nih.gov/): For gene/GOterm related publications
- NCBI (https://www.ncbi.nlm.nih.gov/): For overview and chromosomal information on the gene
- dbPTM (https://awi.cuhk.edu.cn/dbPTM/): For post-translational modifications
- GTEX (https://www.gtexportal.org/home/): For data on expression of the gene in different tissues
- UniProt (“https://www.uniprot.org/”): For information on the protein related to the gene
- Human Protein Atlas (“https://www.proteinatlas.org/”): For information on the protein related to the gene for humans specifically
You can access all of these easily by using one function that uses a
data.frame as input:
# creating a smaller subset for visualization purposes and to keep the main res_de
res_subset <- deresult_to_df(res_macrophage_IFNg_vs_naive, FDR = 0.05)[1:100, ]
buttonifier(
df = res_subset,
col_to_use = "symbol",
create_buttons_to = c("GC", "NCBI", "GTEX", "UNIPROT", "dbPTM", "HPA", "PUBMED"),
ens_col = "id",
ens_species = "Homo_sapiens"
)Again, an overview of the key parameters:
-
df: A data.frame object containing your data. To get one from yourDESeqResultsdata use the function:deresult_to_df(). -
col_to_use: Column where the gene names are stored, default is “SYMBOL”, in this example however the column is named “symbol”. -
create_buttons_to: All of the supported websites. You can pick however many you want. -
ens_col: Where to find the Ensembl IDs in case you want to turn those into buttons too. If not this defaults to NULL and that part is skipped. -
ens_species: The species you are working on. Only needed if you want to turn the Ensembl IDs into buttons.
Importantly, the buttonifier() function directly returns
a DT::datatable by default (not a data.frame).
This is to ensure that the escape = FALSE parameter of
datatable is set and not forgotten as the links/buttons
will not work otherwise (or at least, will displayed very oddly as
“simple text”, not interpreted as the code to generate buttons).
Advanced users that want further customization options to their
datatable can ensure a data.frame is returned
using the output_format parameter (then the
escape = FALSE must be set by hand):
res_subset <- deresult_to_df(res_macrophage_IFNg_vs_naive, FDR = 0.05)[1:100, ]
res_subset <- buttonifier(res_subset,
col_to_use = "symbol",
create_buttons_to = c("GC", "NCBI", "HPA"),
output_format = "DF"
)
DT::datatable(res_subset,
escape = FALSE,
rownames = FALSE,
# other parameters specifically controlling the look of the DT...
options = list(
scrollX = TRUE
)
)As an additional prettifying element, the information on the log2
fold change can be also encoded with small transparent colored bars,
representing the underlying effect sizes. This can be done with the
de_table_painter() function, displayed in the following
chunk:
de_table_painter(res_subset,
rounding_digits = 3,
signif_digits = 5)
## This also works directly on the DESeqResults objects:
de_table_painter(res_macrophage_IFNg_vs_naive[1:50, ],
rounding_digits = 3,
signif_digits = 5)All of the functions included inside the buttonifier()
function are also available as singular functions in case you are only
interested in a subset of them. As a reminder: all functions, except the
one related to the ENSEMBL database, can use/need gene symbols as input,
so that the call to build up the table from its individual columns could
be specified as in the following chunk:
res_subset <- deresult_to_df(res_macrophage_IFNg_vs_naive, FDR = 0.05)[1:100, ]
row.names(res_subset) <- create_link_ENSEMBL(row.names(res_subset), species = "Homo_sapiens")
res_subset$symbol_GC <- create_link_GeneCards(res_subset$symbol)
res_subset$symbol_PubMed <- create_link_PubMed(res_subset$symbol)
res_subset$symbol_NCBI <- create_link_NCBI(res_subset$symbol)
res_subset$symbol_dbPTM <- create_link_dbPTM(res_subset$symbol)
res_subset$symbol_GTEX <- create_link_GTEX(res_subset$symbol)
res_subset$symbol_UniProt <- create_link_UniProt(res_subset$symbol)
res_subset$symbol_HPA <- create_link_HPA(res_subset$symbol)
DT::datatable(res_subset, escape = FALSE,
options = list(
scrollX = TRUE
)
)For information on singular genes you can use:
geneinfo_to_html("IRF1",
res_de = res_macrophage_IFNg_vs_naive,
col_to_use = "symbol"
)Link to the NCBI Gene database: IRF1@NCBI
Link to the GeneCards database: IRF1@GeneCards
Link to the GTEx Portal: IRF1@GTEX
Link to the UniProt Portal: IRF1@UNIPROT
Link to related articles on Pubmed: IRF1@Pubmed
DE p-value (adjusted): 5.580818e-93
DE log2FoldChange: 5.56
It can however also be used without a res_de for a general overview.
geneinfo_to_html("ACTB")Link to the NCBI Gene database: ACTB@NCBI
Link to the GeneCards database: ACTB@GeneCards
Link to the GTEx Portal: ACTB@GTEX
Link to the UniProt Portal: ACTB@UNIPROT
Link to related articles on Pubmed: ACTB@Pubmed
This can be a practical way to generate some HTML content to be embedded e.g. in other contexts such as dashboards, as it is currently implemented in pcaExplorer, ideal and GeneTonic.
More information on GO terms
We display an interactive table for a subset of GO terms - in this case, we select the first 100 rows.
res_enrich_macrophage_topGO$GO.ID <- create_link_GO(res_enrich_macrophage_topGO$GO.ID)
DT::datatable(res_enrich_macrophage_topGO[1:100, ],
escape = FALSE,
options = list(
scrollX = TRUE
)
)Setting escape = FALSE is important here to ensure the
link is turned into a button - since we are dealing with a
datatable where we need to interpret some content directly
as HTML code.
To get information on a singular GO term of interest you can use:
go_to_html("GO:0001525")Pubmed results: angiogenesis@Pubmed
Term: angiogenesis
Ontology: BP
Definition: Blood vessel formation when new vessels emerge from the proliferation of pre-existing blood vessels.
Synonym: blood vessel formation from pre-existing blood vessels
This not only creates a link to the AmiGO database, but also extracts some information about the term itself from the GO.db package.
This approach can be extended to link to additional external resources on genesets, such as MSigDB or Reactome.
Session Info
sessionInfo()
#> R version 4.5.0 (2025-04-11)
#> Platform: aarch64-apple-darwin20
#> Running under: macOS Sonoma 14.7.5
#>
#> Matrix products: default
#> BLAS: /Library/Frameworks/R.framework/Versions/4.5-arm64/Resources/lib/libRblas.0.dylib
#> LAPACK: /Library/Frameworks/R.framework/Versions/4.5-arm64/Resources/lib/libRlapack.dylib; LAPACK version 3.12.1
#>
#> locale:
#> [1] en_US.UTF-8/en_US.UTF-8/en_US.UTF-8/C/en_US.UTF-8/en_US.UTF-8
#>
#> time zone: UTC
#> tzcode source: internal
#>
#> attached base packages:
#> [1] stats4 stats graphics grDevices utils datasets methods
#> [8] base
#>
#> other attached packages:
#> [1] ggplot2_3.5.2
#> [2] TxDb.Hsapiens.UCSC.hg38.knownGene_3.21.0
#> [3] GenomicFeatures_1.61.0
#> [4] topGO_2.59.0
#> [5] SparseM_1.84-2
#> [6] GO.db_3.21.0
#> [7] graph_1.87.0
#> [8] org.Hs.eg.db_3.21.0
#> [9] AnnotationDbi_1.71.0
#> [10] DESeq2_1.49.0
#> [11] SummarizedExperiment_1.39.0
#> [12] Biobase_2.69.0
#> [13] MatrixGenerics_1.21.0
#> [14] matrixStats_1.5.0
#> [15] GenomicRanges_1.61.0
#> [16] GenomeInfoDb_1.45.3
#> [17] IRanges_2.43.0
#> [18] S4Vectors_0.47.0
#> [19] BiocGenerics_0.55.0
#> [20] generics_0.1.4
#> [21] macrophage_1.25.0
#> [22] mosdef_1.5.1
#> [23] BiocStyle_2.37.0
#>
#> loaded via a namespace (and not attached):
#> [1] RColorBrewer_1.1-3 jsonlite_2.0.0 magrittr_2.0.3
#> [4] ggtangle_0.0.6 farver_2.1.2 rmarkdown_2.29
#> [7] BiocIO_1.19.0 fs_1.6.6 ragg_1.4.0
#> [10] vctrs_0.6.5 Rsamtools_2.25.0 memoise_2.0.1
#> [13] RCurl_1.98-1.17 ggtree_3.17.0 progress_1.2.3
#> [16] htmltools_0.5.8.1 S4Arrays_1.9.0 curl_6.2.2
#> [19] SparseArray_1.9.0 gridGraphics_0.5-1 sass_0.4.10
#> [22] bslib_0.9.0 htmlwidgets_1.6.4 desc_1.4.3
#> [25] httr2_1.1.2 plyr_1.8.9 cachem_1.1.0
#> [28] GenomicAlignments_1.45.0 igraph_2.1.4 lifecycle_1.0.4
#> [31] pkgconfig_2.0.3 Matrix_1.7-3 R6_2.6.1
#> [34] fastmap_1.2.0 gson_0.1.0 digest_0.6.37
#> [37] aplot_0.2.5 enrichplot_1.29.1 colorspace_2.1-1
#> [40] patchwork_1.3.0 goseq_1.61.0 crosstalk_1.2.1
#> [43] textshaping_1.0.1 RSQLite_2.3.11 labeling_0.4.3
#> [46] filelock_1.0.3 mgcv_1.9-3 httr_1.4.7
#> [49] polyclip_1.10-7 abind_1.4-8 compiler_4.5.0
#> [52] bit64_4.6.0-1 withr_3.0.2 BiocParallel_1.43.0
#> [55] DBI_1.2.3 BiasedUrn_2.0.12 ggforce_0.4.2
#> [58] R.utils_2.13.0 biomaRt_2.65.0 MASS_7.3-65
#> [61] geneLenDataBase_1.45.0 rappdirs_0.3.3 DelayedArray_0.35.1
#> [64] rjson_0.2.23 tools_4.5.0 ape_5.8-1
#> [67] R.oo_1.27.1 glue_1.8.0 restfulr_0.0.15
#> [70] nlme_3.1-168 GOSemSim_2.35.0 grid_4.5.0
#> [73] reshape2_1.4.4 fgsea_1.35.0 gtable_0.3.6
#> [76] R.methodsS3_1.8.2 tidyr_1.3.1 hms_1.1.3
#> [79] data.table_1.17.2 xml2_1.3.8 XVector_0.49.0
#> [82] ggrepel_0.9.6 pillar_1.10.2 stringr_1.5.1
#> [85] yulab.utils_0.2.0 splines_4.5.0 dplyr_1.1.4
#> [88] tweenr_2.0.3 BiocFileCache_2.99.0 treeio_1.33.0
#> [91] lattice_0.22-7 rtracklayer_1.69.0 bit_4.6.0
#> [94] tidyselect_1.2.1 locfit_1.5-9.12 Biostrings_2.77.0
#> [97] knitr_1.50 bookdown_0.43 xfun_0.52
#> [100] DT_0.33 stringi_1.8.7 UCSC.utils_1.5.0
#> [103] lazyeval_0.2.2 ggfun_0.1.8 yaml_2.3.10
#> [106] evaluate_1.0.3 codetools_0.2-20 tibble_3.2.1
#> [109] qvalue_2.41.0 BiocManager_1.30.25 ggplotify_0.1.2
#> [112] cli_3.6.5 systemfonts_1.2.3 jquerylib_0.1.4
#> [115] Rcpp_1.0.14 dbplyr_2.5.0 png_0.1-8
#> [118] XML_3.99-0.18 parallel_4.5.0 pkgdown_2.1.2.9000
#> [121] blob_1.2.4 prettyunits_1.2.0 clusterProfiler_4.17.0
#> [124] DOSE_4.3.0 bitops_1.0-9 txdbmaker_1.5.1
#> [127] tidytree_0.4.6 scales_1.4.0 purrr_1.0.4
#> [130] crayon_1.5.3 rlang_1.1.6 cowplot_1.1.3
#> [133] fastmatch_1.1-6 KEGGREST_1.49.0